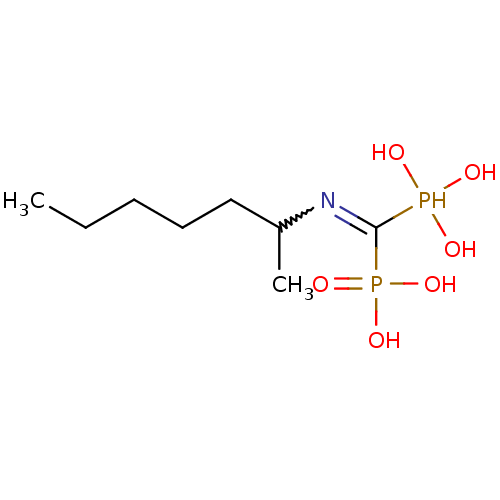
BDBM50135816 CHEMBL54480::[(1-Methyl-hexylamino)-phosphono-methyl]-phosphonic acid
SMILES CCCCCC(C)N=C(P(O)(O)O)P(O)(O)=O
InChI Key InChIKey=PFEHXKXBMJQUCZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50135816
Found 4 hits for monomerid = 50135816
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 110nMAssay Description:Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligandMore data for this Ligand-Target Pair
TargetPolyprenyl synthetase family protein(Plasmodium falciparum (isolate 3D7))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataIC50: 1.14E+3nMAssay Description:Inhibitory activity against Leishmania major Farnesyl diphosphate synthaseMore data for this Ligand-Target Pair
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataIC50: 1.15E+3nMAssay Description:Inhibitory activity against farnesyl Pyrophosphate Synthase expressed as #NAME? (M)More data for this Ligand-Target Pair
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibitory activity against farnesyl Pyrophosphate Synthase was determinedMore data for this Ligand-Target Pair