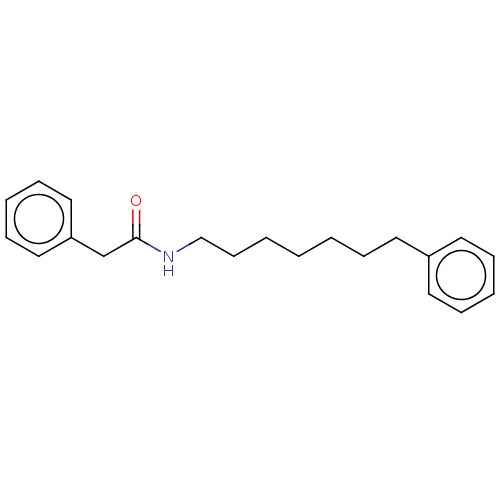
BDBM50150633 CHEMBL3769407
SMILES O=C(Cc1ccccc1)NCCCCCCCc1ccccc1
InChI Key InChIKey=SOAWUVOKDMVYCR-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50150633
Found 3 hits for monomerid = 50150633
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Sapienza University Of Rome
Curated by ChEMBL
Sapienza University Of Rome
Curated by ChEMBL
Affinity DataIC50: 1.21E+3nMAssay Description:Antagonist activity at recombinant human TRPV1 channel expressed in HEK293 cells assessed as inhibition of capsiacin-induced Ca2+ flux preincubated f...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Sapienza University Of Rome
Curated by ChEMBL
Sapienza University Of Rome
Curated by ChEMBL
Affinity DataEC50: 660nMAssay Description:Agonist activity at recombinant human TRPV1 channel expressed in HEK293 cells assessed as increase in intracellular Ca2+ concentration by Fluo-4-AM d...More data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1 [30-579](Rattus norvegicus (rat))
Sapienza University Of Rome
Curated by ChEMBL
Sapienza University Of Rome
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of rat brain FAAH assessed as hydrolysis of [14C]AEA to [14C]Ethanolamine incubated for 30 mins by scintillation counting methodMore data for this Ligand-Target Pair