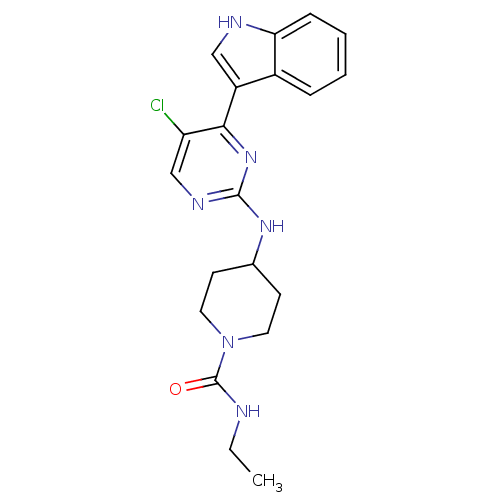
BDBM50211424 4-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-ylamino)-N-ethylpiperidine-1-carboxamide::4-{[5-chloro-4-(1H-indol-3-yl)pyrimidin-2-yl]amino}-N-ethylpiperidine-1-carboxamide::CHEMBL248176
SMILES CCNC(=O)N1CCC(CC1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12
InChI Key InChIKey=ARMFMDYRYOKSOW-UHFFFAOYSA-N
Data 5 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50211424
Found 5 hits for monomerid = 50211424
Affinity DataIC50: 704nMAssay Description:Inhibition of cJun translocation in IL-1-beta-stimulated A549 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.52E+3nMAssay Description:Inhibition of CDK2 by IMAP technologyMore data for this Ligand-Target Pair