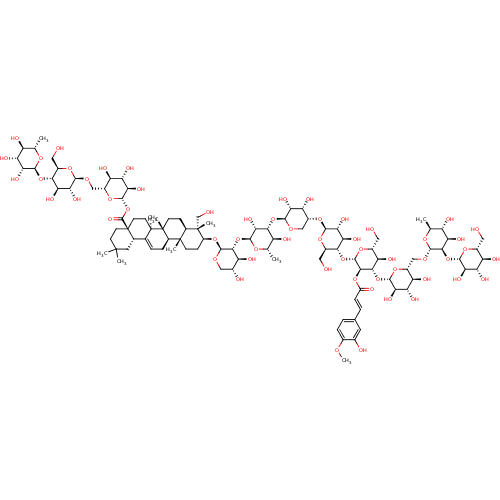
BDBM50322754 3-O-beta-Dglucopyranosyl-(1-2)-alpha-L-rhamnopyranosyl-(1-6)-beta-D-glucopyranosyl-(1-3)-[(2-O-isoferuloyl)-beta-D-glucopyranosyl]-(1-4)-beta-D-glucopyranosyl-(1-4)-beta-D-ribopyranosyl-(1-3)-alpha-Lrhamnopyranosyl-(1-2)-alpha-L-arabinopyranosyl hederagenin28-O-alpha-L-rhamnopyranosyl-(1-4)-beta-D-glucopyranosyl-(1-6)-beta-D-glucopyranoside::CHEMBL1171251
SMILES COc1ccc(\C=C\C(=O)O[C@H]2[C@H](O[C@@H]3[C@@H](CO)O[C@@H](O[C@@H]4CO[C@@H](O[C@@H]5[C@@H](O)[C@H](C)O[C@@H](O[C@@H]6[C@@H](O)[C@H](O)CO[C@H]6O[C@H]6CC[C@@]7(C)[C@@H](CC[C@]8(C)[C@@H]7CC=C7[C@@H]9CC(C)(C)CC[C@@]9(CC[C@@]87C)C(=O)O[C@@H]7O[C@H](CO[C@@H]8O[C@H](CO)[C@@H](O[C@@H]9O[C@@H](C)[C@H](O)[C@@H](O)[C@H]9O)[C@H](O)[C@H]8O)[C@@H](O)[C@H](O)[C@H]7O)[C@]6(C)CO)[C@@H]5O)[C@H](O)[C@@H]4O)[C@H](O)[C@H]3O)O[C@H](CO)[C@@H](O)[C@@H]2O[C@@H]2O[C@H](CO[C@@H]3O[C@@H](C)[C@H](O)[C@@H](O)[C@H]3O[C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)[C@@H](O)[C@H](O)[C@H]2O)cc1O
InChI Key InChIKey=FLILBXBCHAHNMP-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50322754
Found 2 hits for monomerid = 50322754