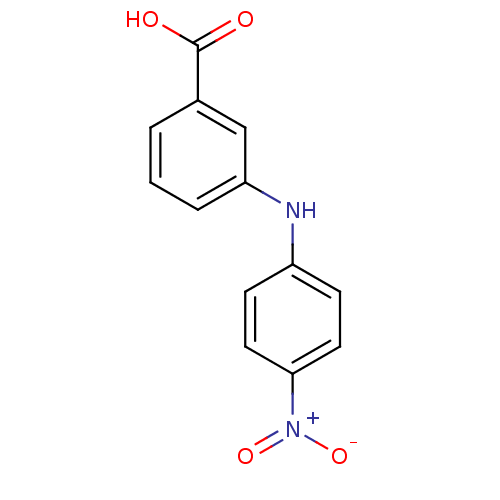
BDBM50337281 3-[N-(4-nitrophenyl)amino]benzoic acid::CHEMBL1682200::US9271961, 5
SMILES OC(=O)c1cccc(Nc2ccc(cc2)[N+]([O-])=O)c1
InChI Key InChIKey=YRZASYCIZSWCKB-UHFFFAOYSA-N
Data 21 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 21 hits for monomerid = 50337281
Found 21 hits for monomerid = 50337281
Affinity DataIC50: 30nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Inhibition of AKR1C3 by fluorimetric methodMore data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataIC50: 740nMAssay Description:Inhibition of COX2 expressed in baculovirus infected SF-21 cells assessed as formation of PGH2 from PGG2 using arachidonic acid as substrate preincub...More data for this Ligand-Target Pair
Affinity DataIC50: 740nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataIC50: 740nMAssay Description:Inhibition of recombinant COX2More data for this Ligand-Target Pair
Affinity DataIC50: 3.38E+3nMAssay Description:Inhibition of recombinant AKR1C2 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+3nMAssay Description:Inhibition of AKR1C2 by fluorimetric methodMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Human)
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 6.74E+3nMAssay Description:Inhibition of recombinant AKR1C1 assessed as enzyme catalyzed oxidation of S-tetralol by fluorimetric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Human)
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 6.74E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Human)
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 6.74E+3nMAssay Description:Inhibition of recombinant AKR1C1 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Human)
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 3.08E+4nMAssay Description:Inhibition of recombinant COX1More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Human)
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 3.08E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C4(Human)
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 3.27E+4nMAssay Description:Inhibition of recombinant AKR1C4 assessed as enzyme catalyzed oxidation of S-tetralol by fluorimetric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C4(Human)
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 3.27E+4nMAssay Description:Inhibition of recombinant AKR1C4 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C4(Human)
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 3.27E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.60E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.60E+4nMAssay Description:Inhibition of recombinant AKR1B10 assessed as NADP+ dependent reduction of DL-glyceraldehyde by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of recombinant AKR1B1 assessed as NADP+ dependent reduction of DL-glyceraldehyde by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair