BDBM50467836 CHEMBL4282264
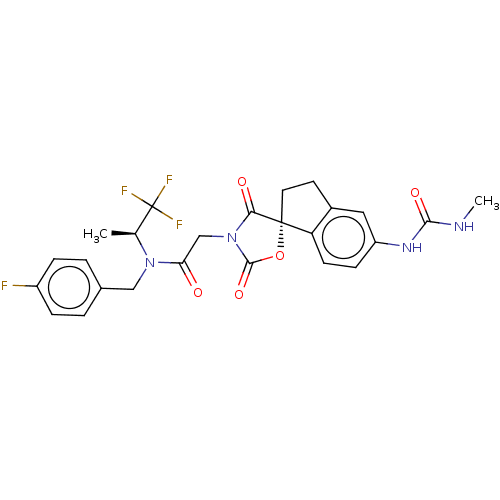
SMILES C[C@@H](C(F)(F)F)N(Cc1ccc(cc1)F)C(=O)CN2C(=O)[C@]3(CCc4c3ccc(c4)NC(=O)NC)OC2=O
InChI Key InChIKey=VRVJKILQRBSEAG-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 100 hits for monomerid = 50467836
Found 100 hits for monomerid = 50467836
Affinity DataKd: 0.00128nMAssay Description:Binding affinity to human Biotin-Avi-P300 assessed as dissociation constant using Neutravidin as ligand measured by Surface Plasmon Resonance methodMore data for this Ligand-Target Pair
Affinity DataKd: 1.40nMAssay Description:Binding affinity to human Biotin-Avi-P300 using Neutravidin as ligand measured by Surface Plasmon Resonance methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:Inhibition of CBP BHC domain (unknown origin) by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:Inhibition of CBP-BHC domain (unknown origin) assessed as measuring acetylation of biotinylated histone H4 peptide by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:Inhibition of N-terminal 6His-Flag tagged CBP-BHC domain (1072 to 1859 residues) (unknown origin) expressed in Sf9 cells using the Bac-to-Bac baculov...More data for this Ligand-Target Pair
Affinity DataIC50: 9.60nMAssay Description:Inhibition of P300 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 9.80nMAssay Description:Inhibition of p300-BHC domain (unknown origin) assessed as measuring acetylation of biotinylated histone H4 peptide by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9.80nMAssay Description:Inhibition of N-terminal 6His-Flag tagged p300-BHC domain (1036 to 1822 residues) (unknown origin) expressed in Sf9 cells using the Bac-to-Bac baculo...More data for this Ligand-Target Pair
Affinity DataKd: 15nMAssay Description:Binding affinity to recombinant human p300 HAT domain expressed in Escherichia coli BL21-CodonPlus(DE3)-RIL cells assessed as dissociation constant u...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of p300 (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scint...More data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:Inhibition of p300 HAT (unknown origin) using 3H-acetyl-CoA as substrate assessed as H3 peptide acetylation preincubated with compound for 15 mins fo...More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of p300 HAT domain (unknown origin) using Biotin-C6-GRGKGGKGLGKGGAK as substrate pretreated with enzyme for 30 mins followed by incubation...More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of recombinant human N-terminal 6His-FLAG-tagged p300 BHC domain (1036 to 1822 residues) expressed in baculovirus infected Sf9 insect cell...More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of human P300 bromodomain HAT-C/H3 domain using histone as substrate in presence of acetyl-CoA by TR-FRET analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of human recombinant p300 HAT domain using histone H4 peptide and acetyl-coA as substrates preincubated for 30 mins followed by substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of p300 HAT domain (unknown origin) using Biotin-C6-GRGKGGKGLGKGGAK as substrate pretreated with enzyme for 30 mins followed by incubation...More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of p300 HAT domain (unknown origin) preincubated for 30 mins followed by histone H4 Peptide (Biotin-C6-GRGKGGKGLGKGGAK) and acetyl coenzym...More data for this Ligand-Target Pair
Affinity DataIC50: 61nMAssay Description:Inhibition of CBP (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scinti...More data for this Ligand-Target Pair
Affinity DataIC50: 67nMAssay Description:Inhibition of p300 (unknown origin) using histone H3 (1 to 21 residues) as substrate preincubated for 15 mins followed by substrate and [3H]acetyl-Co...More data for this Ligand-Target Pair
Affinity DataEC50: 73nMAssay Description:Inhibition of p300/CBP in human PC-3 cells assessed as reduction in H3K27Ac level incubated for 3 hrs by Hoechst 33342 staining based high content mi...More data for this Ligand-Target Pair
Affinity DataEC50: 175nMAssay Description:Inhibition of p300/CBP in human PC-3 cells assessed as reduction in H3K18Ac level incubated for 3 hrs by Hoechst 33342 staining based high content mi...More data for this Ligand-Target Pair
Affinity DataIC50: 2.69E+3nMAssay Description:Inhibition of Plk3 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of TrkC (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of TrkB (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of TrkA (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of TYRO3 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of ZIPK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Wee1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Inhibition of p300/CBP in human PC-3 cells assessed as reduction in H3K9Ac level incubated for 3 hrs by Hoechst 33342 staining based high content mic...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of BRDT (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of BRD4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of BRD3 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Aurora1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Akt1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Abl (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of ALK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Fyn (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Flt1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of FGFR1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of FAK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of IGF1R (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Gsk3beta (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Gsk3alpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of GRK5 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of JAK3 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of JAK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of InsR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of IKKE (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of LTK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Kdr (unknown origin)More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)