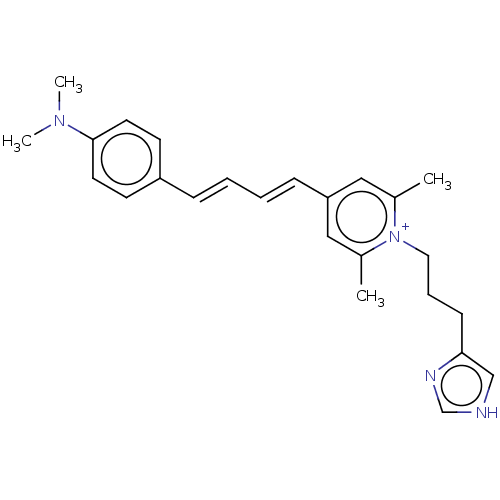
BDBM50538676 CHEMBL4636487
SMILES OC(=O)C(F)(F)F.[O-]C(=O)C(F)(F)F.CN(C)c1ccc(\C=C\C=C\c2cc(C)[n+](CCCc3c[nH]cn3)c(C)c2)cc1
InChI Key InChIKey=AQJCVXSDQBVZED-UHFFFAOYSA-M
Data 2 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50538676
Found 2 hits for monomerid = 50538676
Affinity DataKi: 12nMAssay Description:Displacement of [3H]-UR-PI294 from Galphai2/Gbeta1gamma2-coupled human recombinant H3R expressed in baculovirus infected Sf9 insect cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 589nMAssay Description:Displacement of [3H]-histamine from Galphai2/Gbeta1gamma2-coupled human recombinant H4R expressed in baculovirus infected Sf9 insect cell membranesMore data for this Ligand-Target Pair