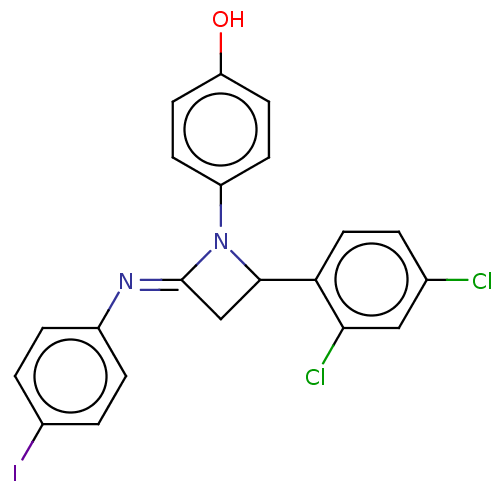
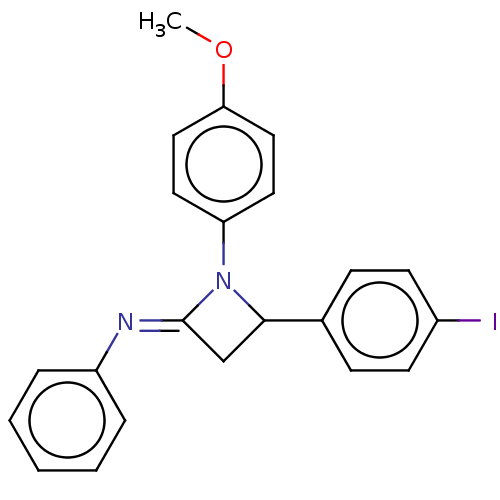
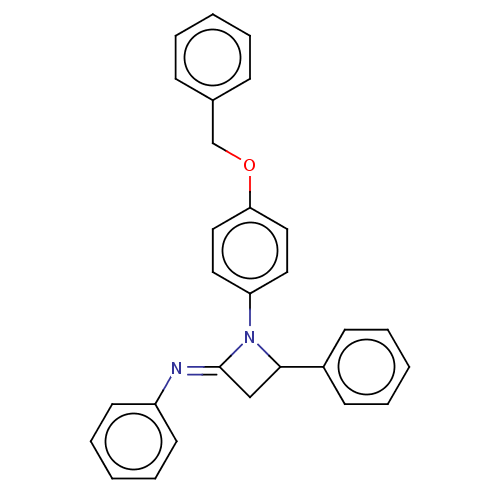
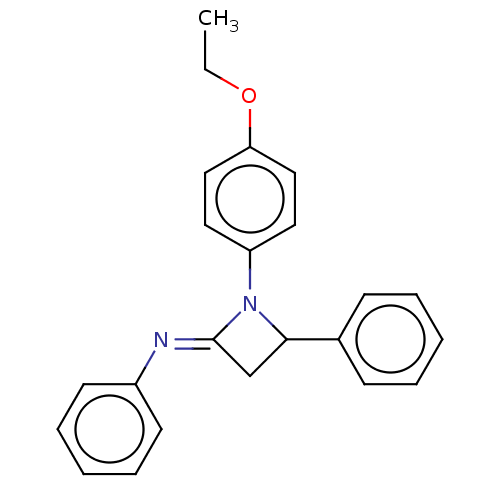
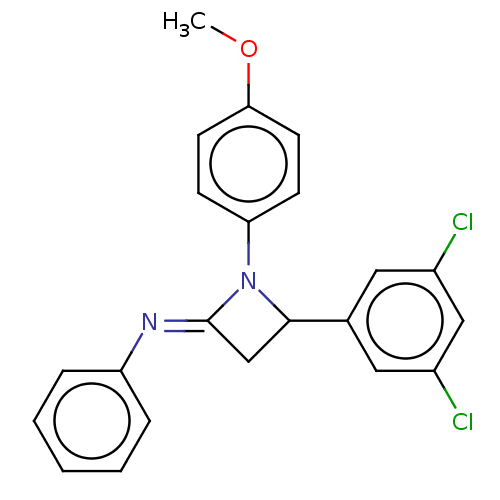
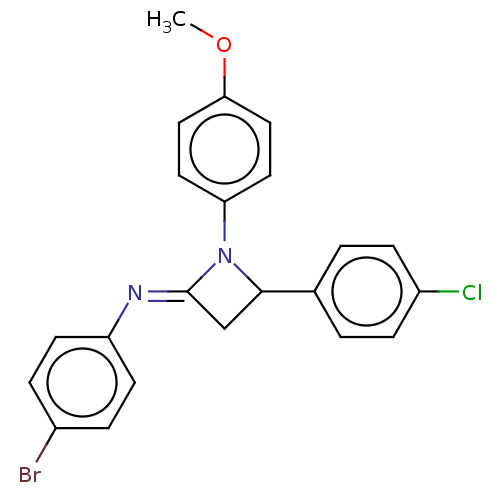
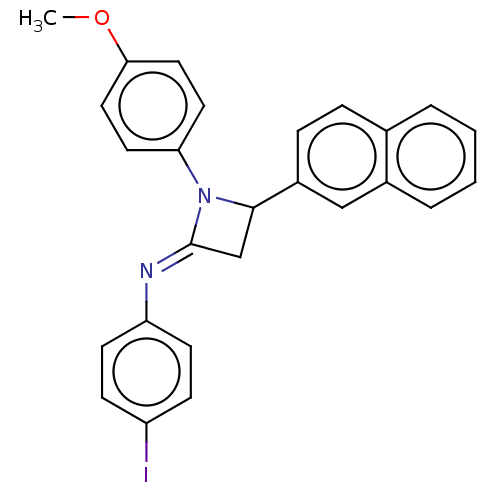
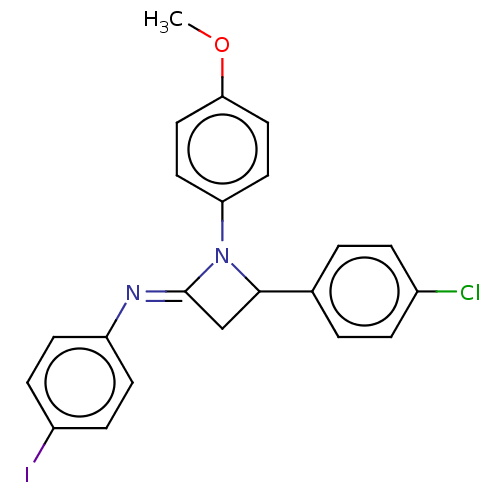
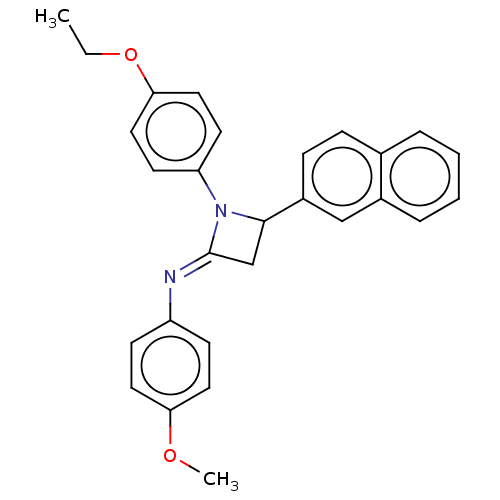
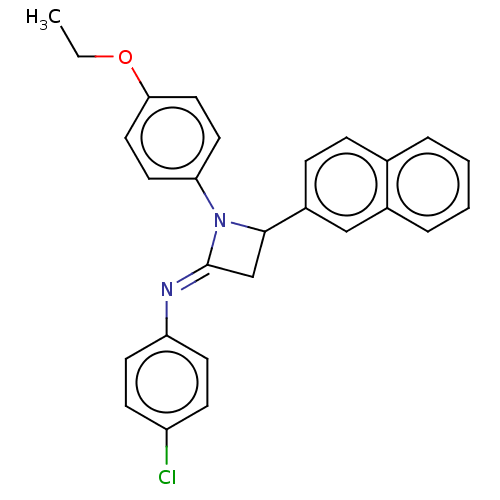
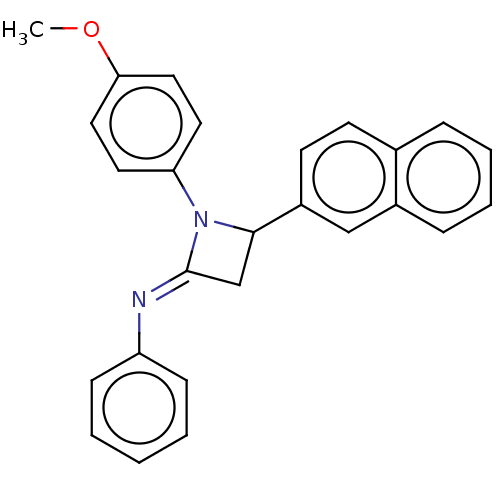
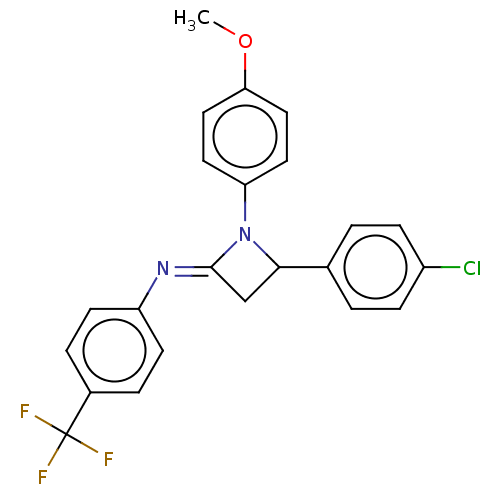
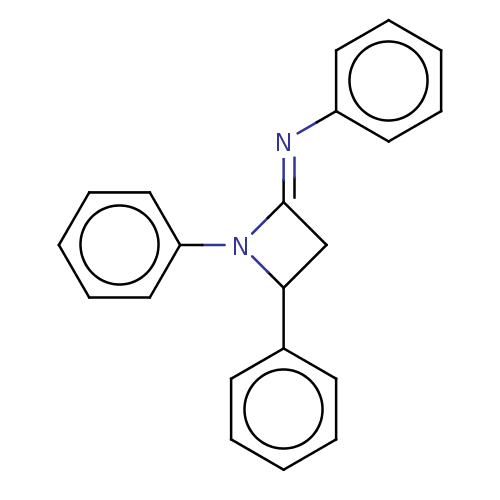
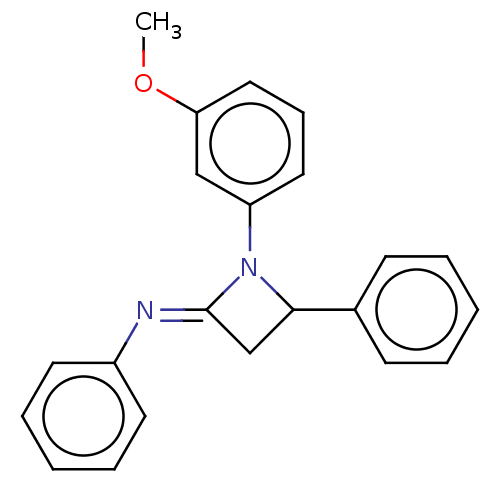
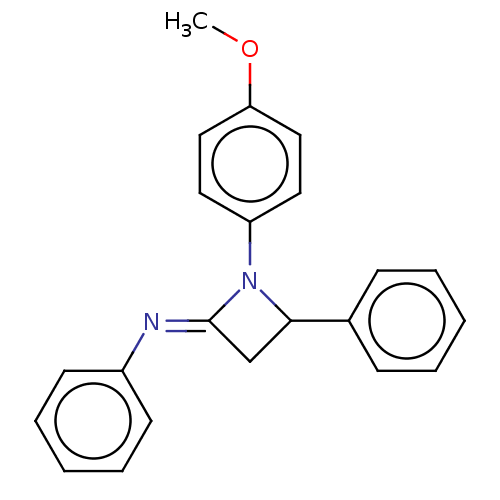
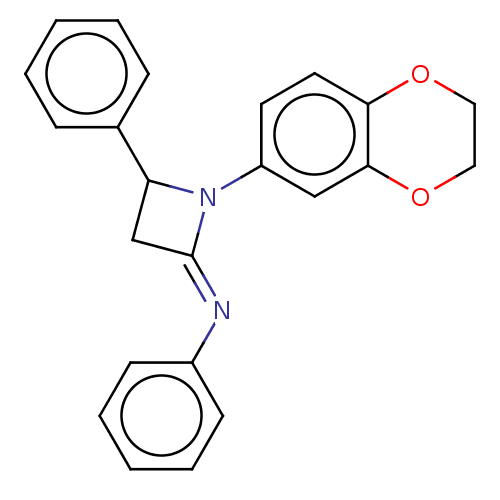
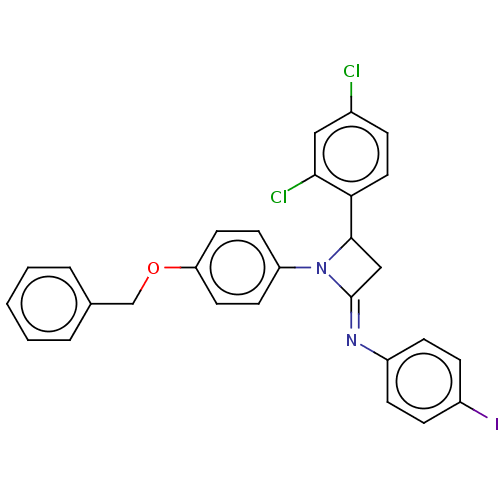
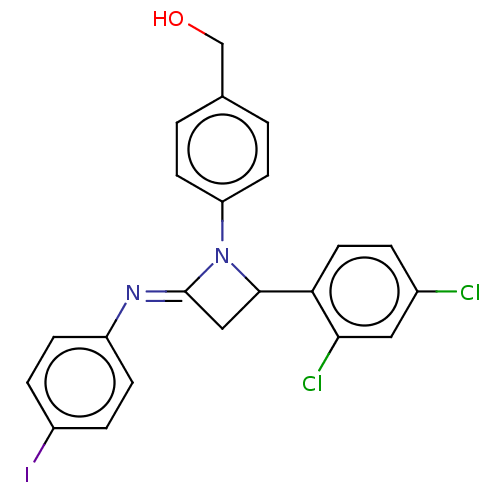
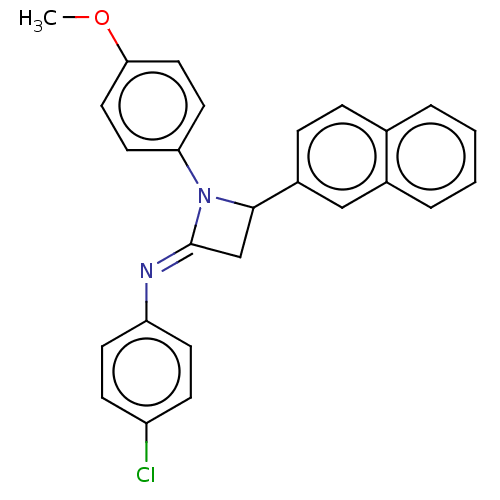
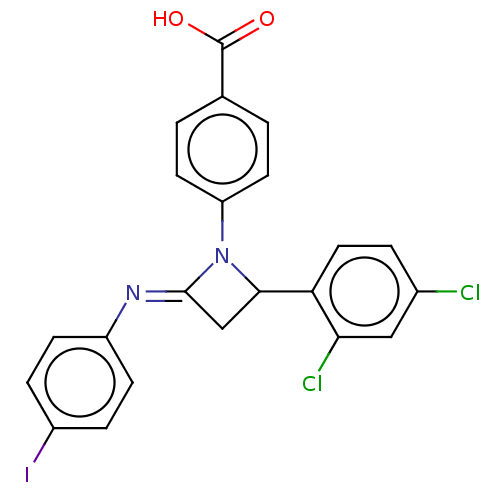
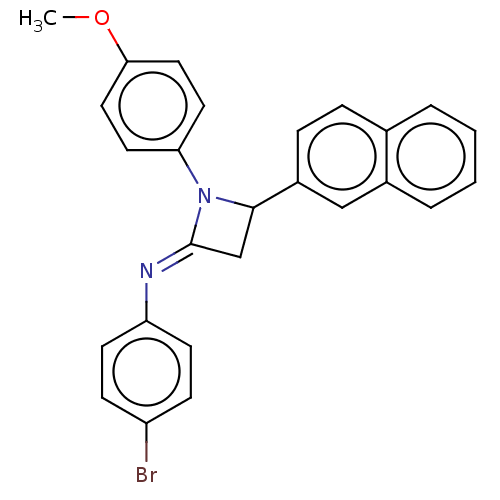
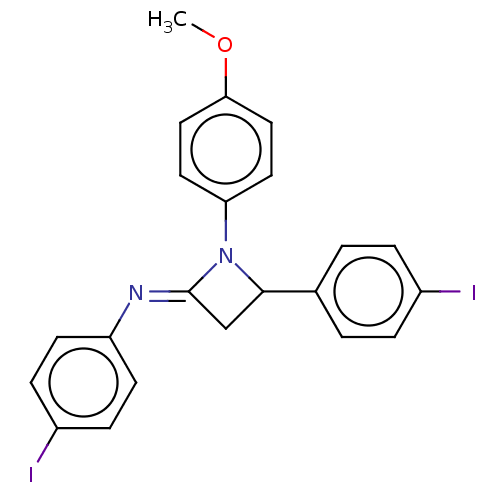
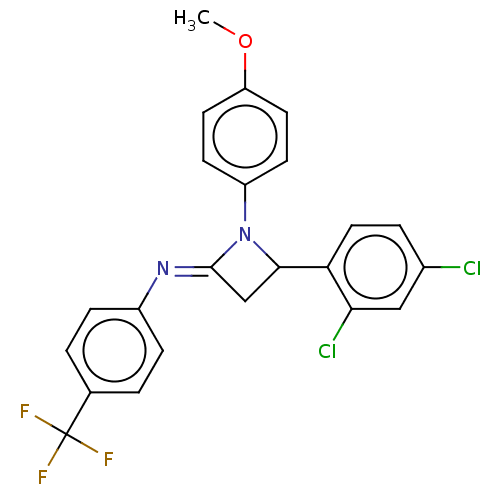
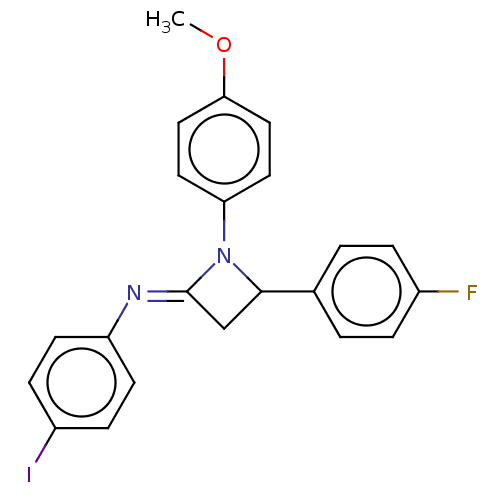
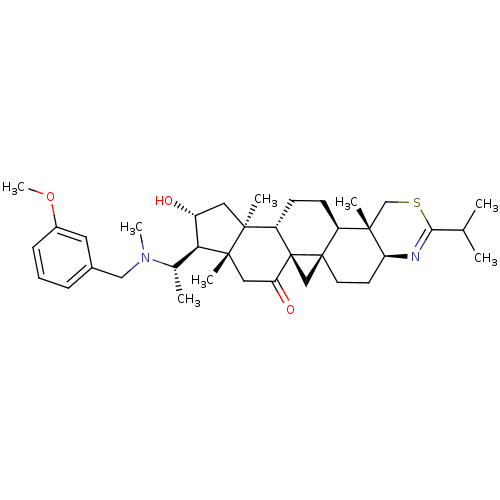
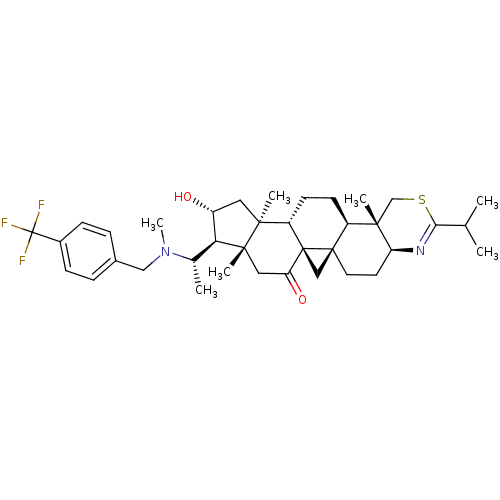
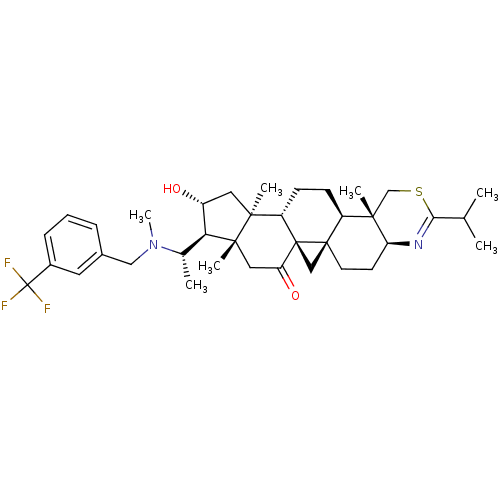
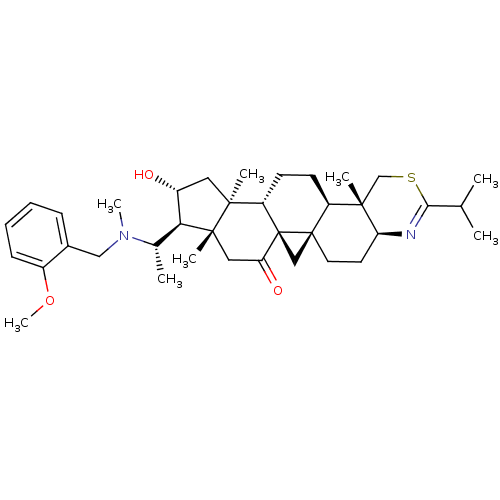
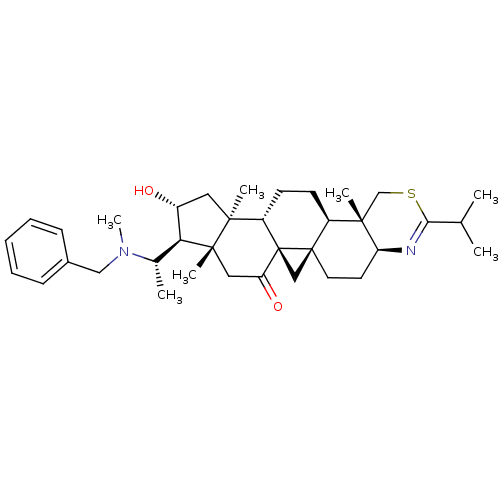
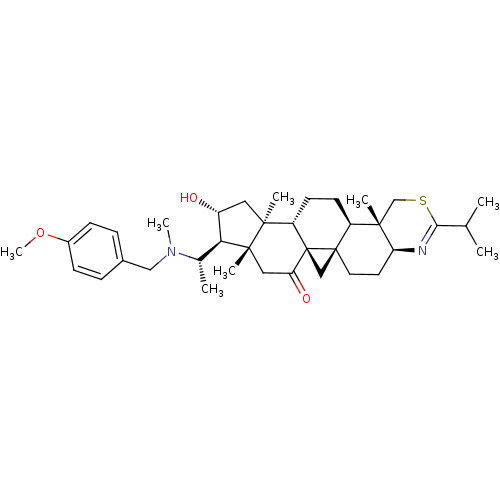
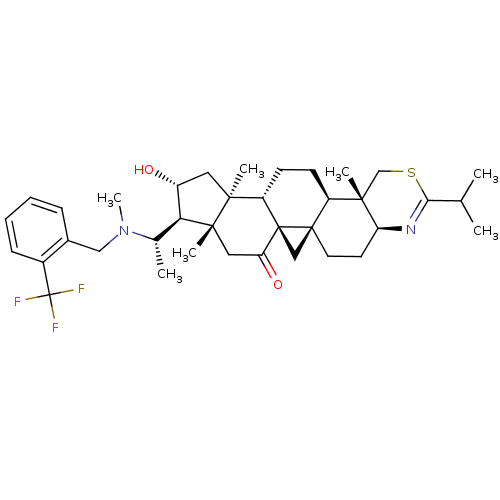
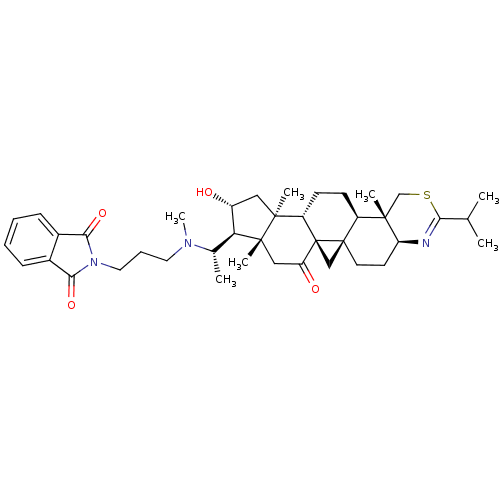
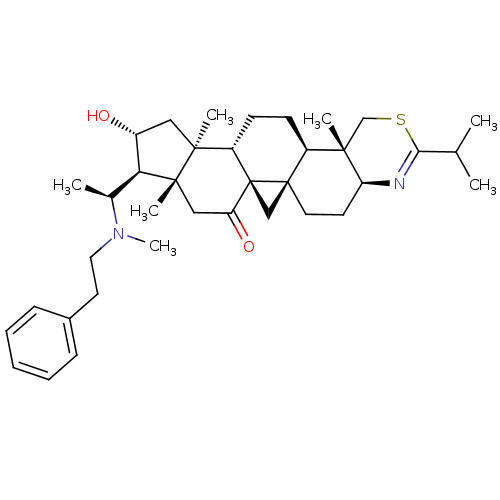
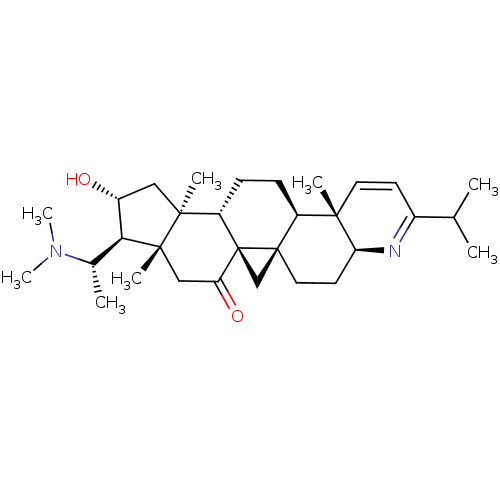
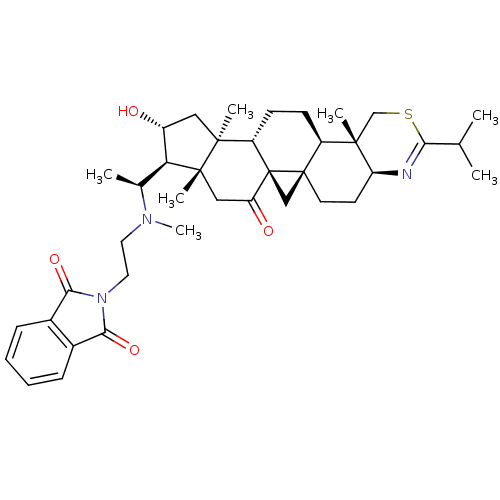
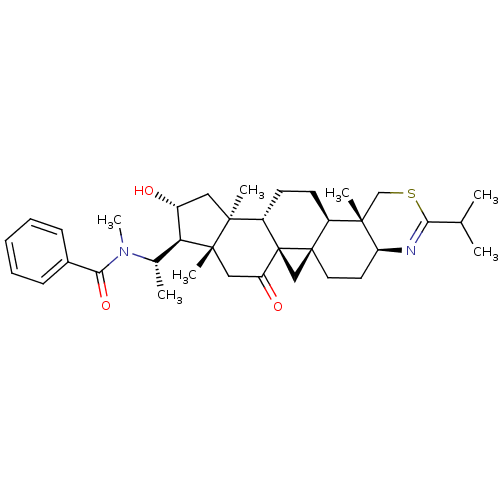
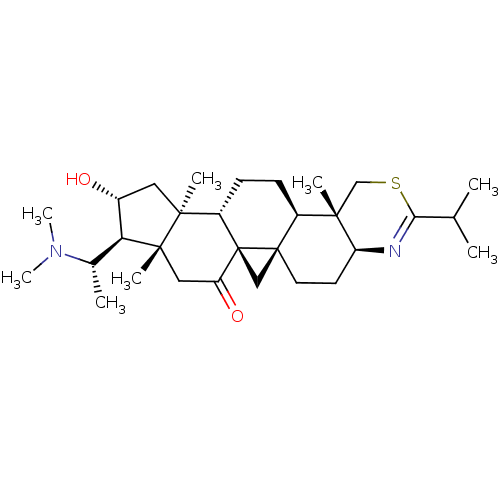
Affinity DataKi: 280nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
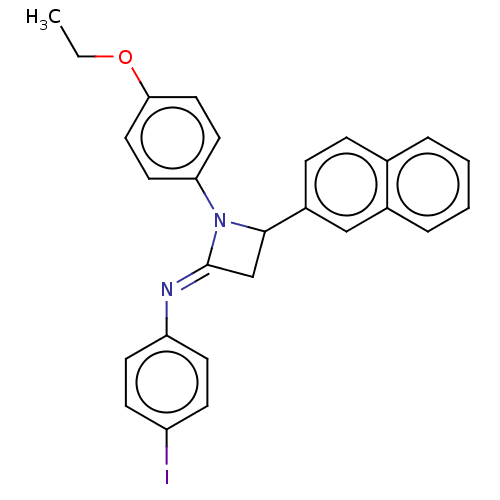
Affinity DataKi: 730nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
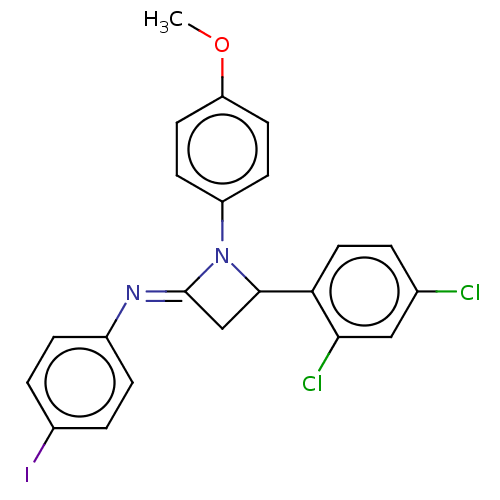
Affinity DataKi: 1.15E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
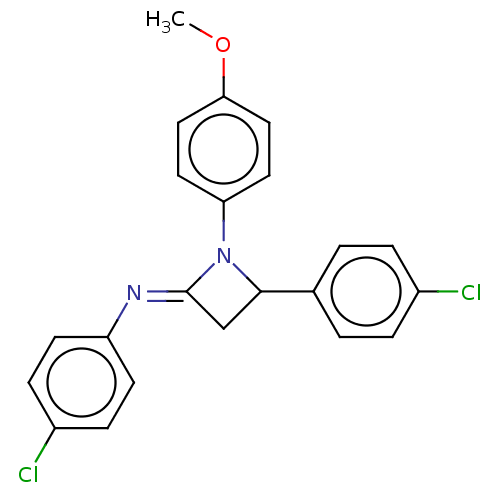
Affinity DataKi: 1.40E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.55E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.58E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.77E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.81E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.84E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.89E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.93E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.96E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.20E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.36E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.41E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.69E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.89E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.94E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 4.15E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5.23E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5.66E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 6.04E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 6.49E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans methodMore data for this Ligand-Target Pair