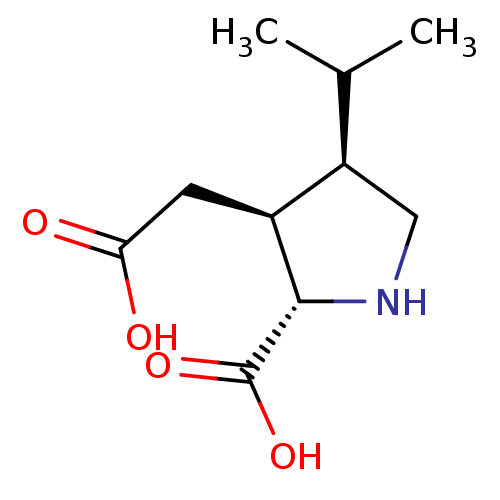
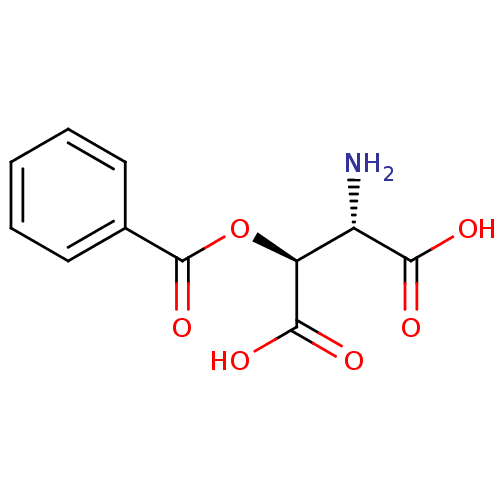
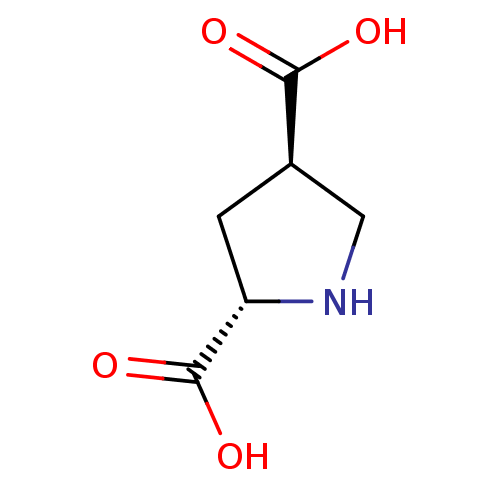
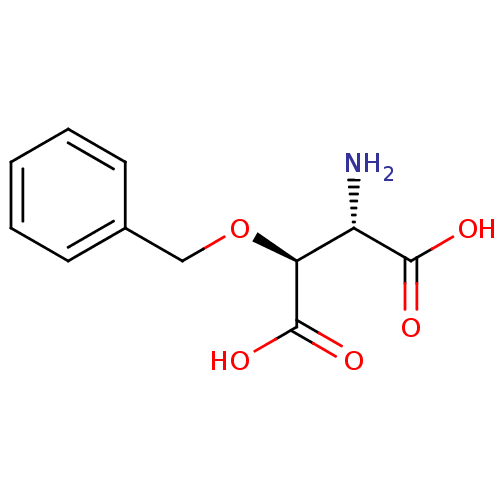
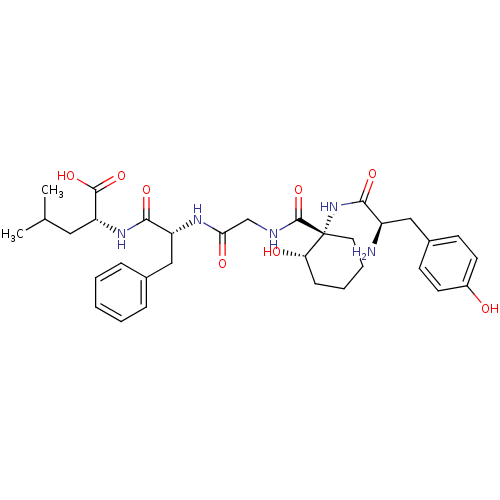
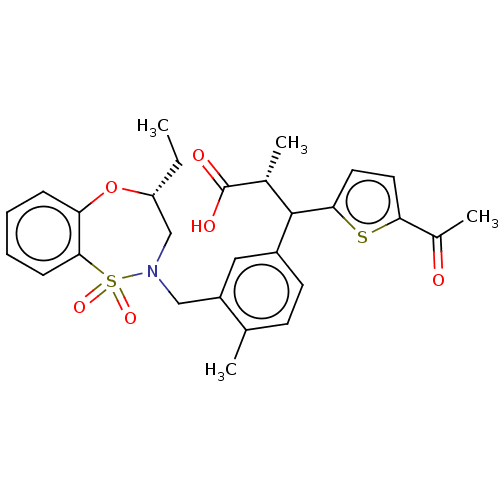
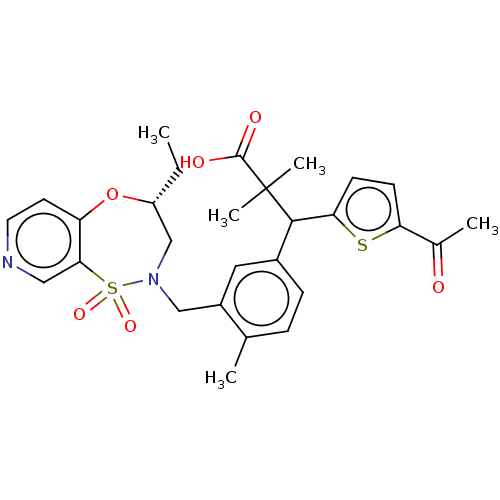
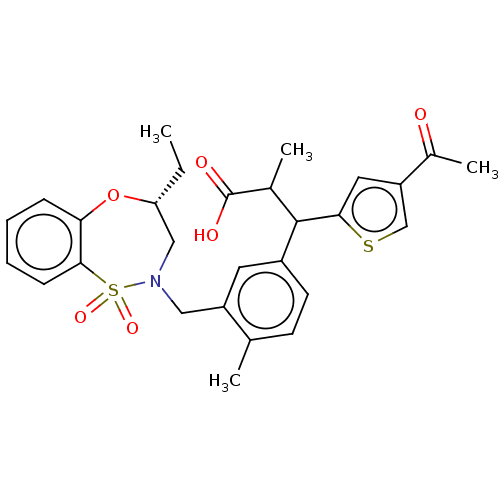
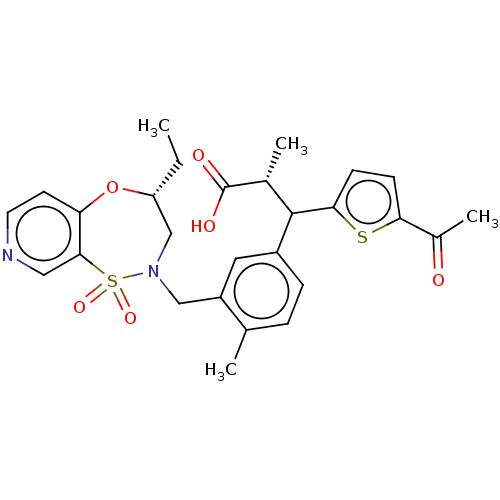
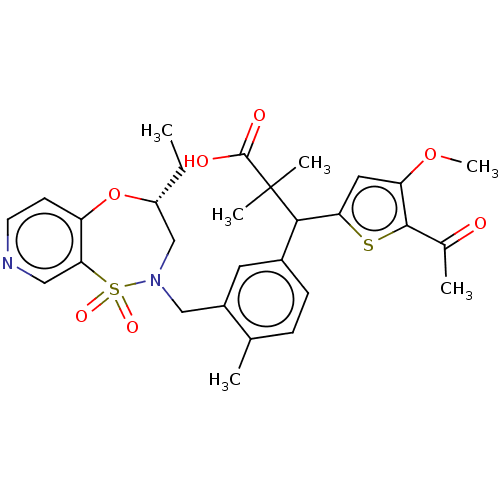
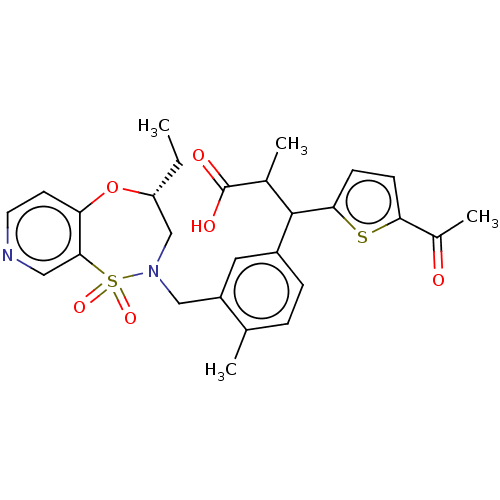
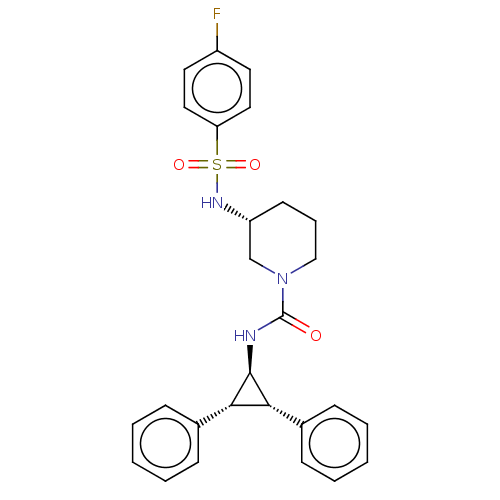
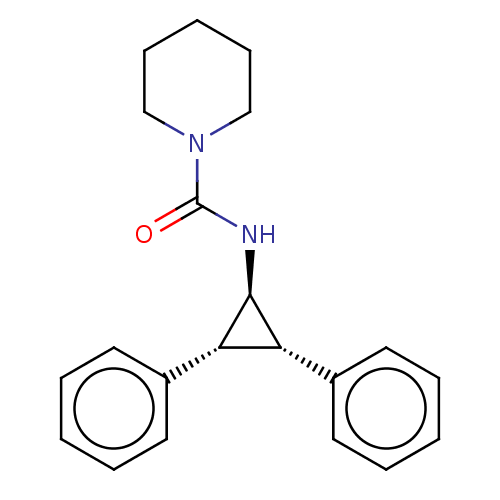
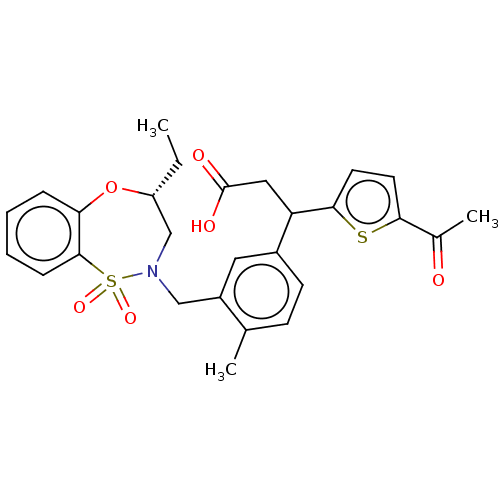
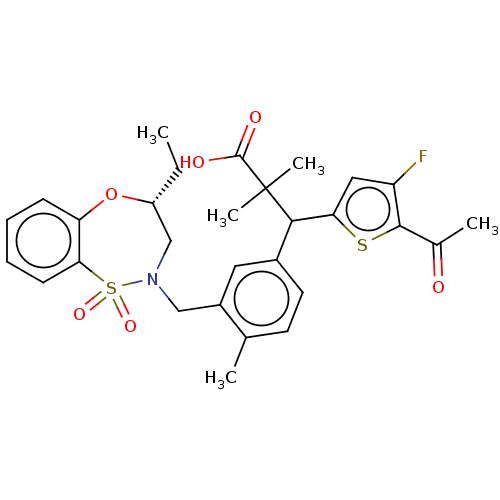
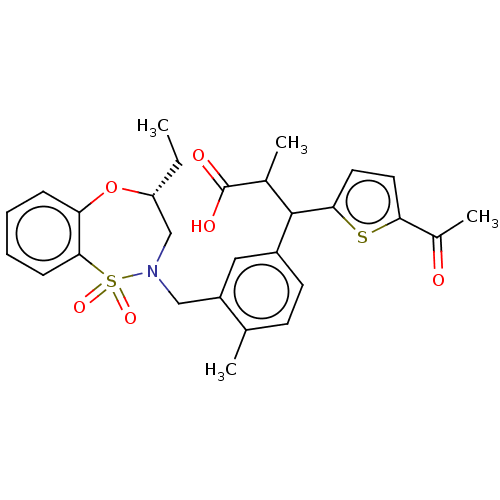
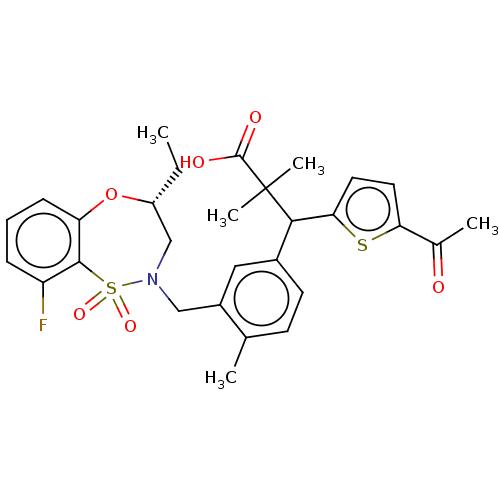
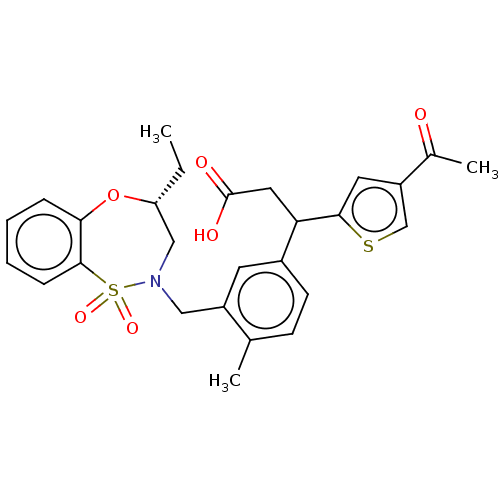
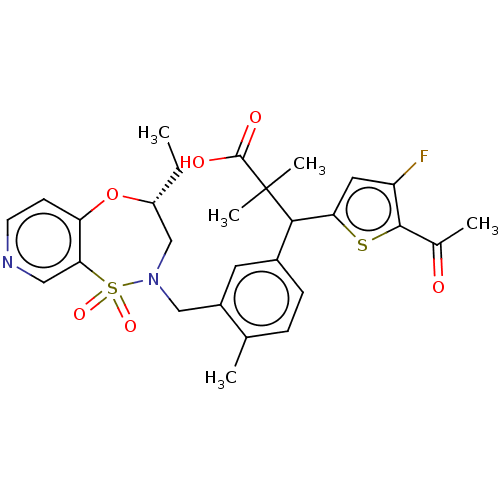
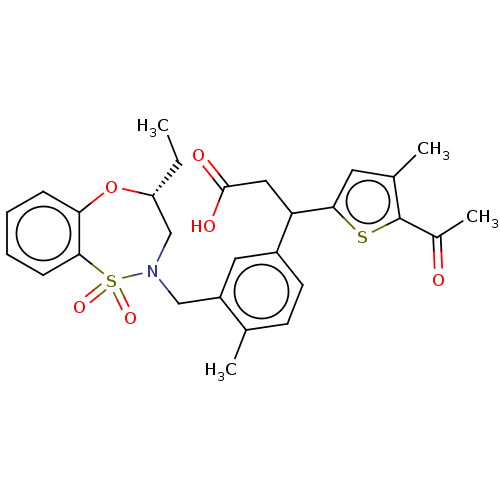
Report error Found 513 with Last Name = 'nakajima' and Initial = 't'
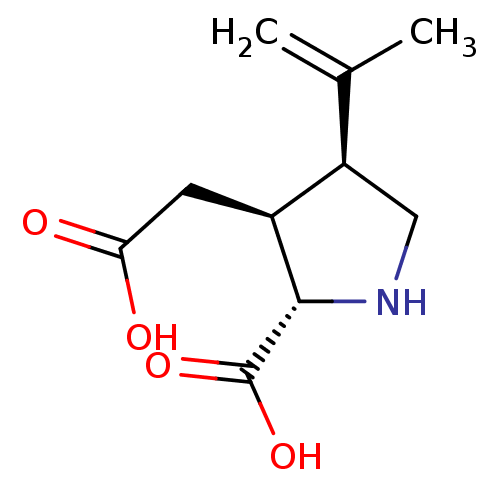
TargetGlutamate receptor ionotropic, kainate 1(Rat)
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
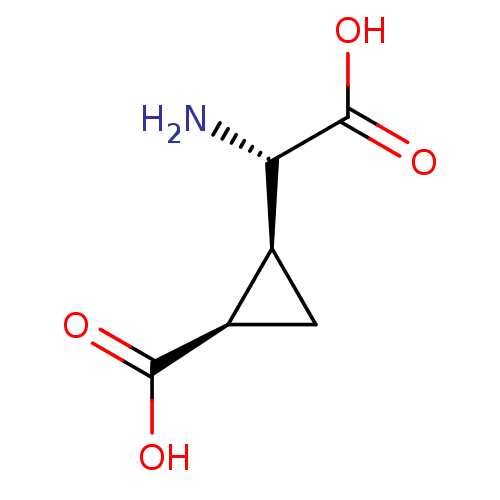
TargetGlutamate receptor ionotropic, NMDA 1(Rat)
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
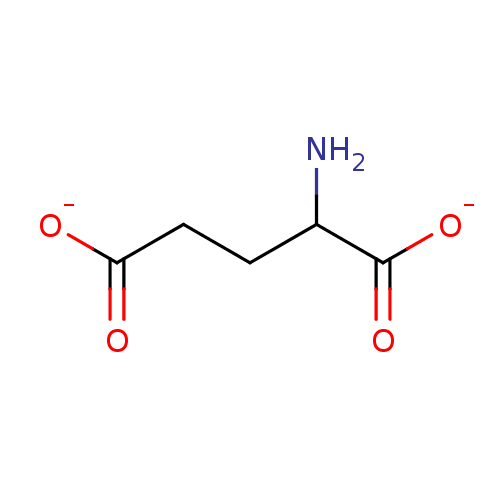
TargetGlutamate receptor ionotropic, kainate 1(Rat)
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
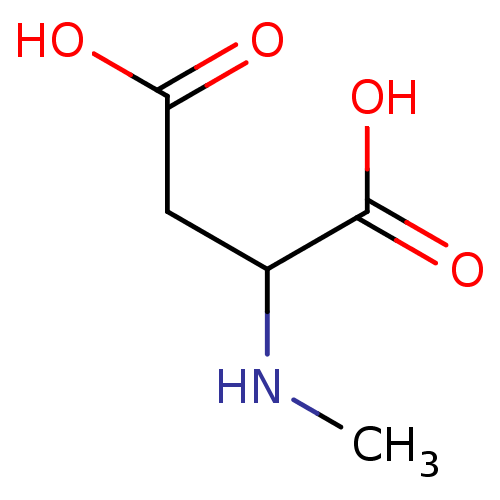
TargetGlutamate receptor ionotropic, NMDA 1(Rat)
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
TargetGlutamate receptor ionotropic, NMDA 1(Rat)
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
TargetGlutamate receptor ionotropic, kainate 1(Rat)
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
TargetGlutamate receptor ionotropic, kainate 1(Rat)
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
TargetGlutamate receptor ionotropic, kainate 1(Rat)
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
TargetGlutamate receptor ionotropic, kainate 1(Rat)
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
TargetGlutamate receptor ionotropic, NMDA 1(Rat)
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
TargetGlutamate receptor ionotropic, NMDA 1(Rat)
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
TargetGlutamate receptor ionotropic, NMDA 1(Rat)
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
TargetGlutamate receptor ionotropic, NMDA 1(Rat)
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
TargetGlutamate receptor ionotropic, NMDA 1(Rat)
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
TargetGlutamate receptor ionotropic, kainate 1(Rat)
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
Suntory Institute For Bioorganic Research
Curated by PDSP Ki Database
Affinity DataIC50: 0.00500nMAssay Description:Inhibitory activity against forskolin-stimulated cAMP production in chinese hamster ovary cells expressing Opioid receptor delta 1More data for this Ligand-Target Pair
Affinity DataIC50: 0.0100nMAssay Description:Inhibitory activity against forskolin-stimulated cAMP production in chinese hamster ovary cells expressing Opioid receptor delta 1More data for this Ligand-Target Pair
Affinity DataIC50: 0.0100nMAssay Description:Inhibitory activity against forskolin-stimulated cAMP production in chinese hamster ovary cells expressing Opioid receptor delta 1More data for this Ligand-Target Pair
Affinity DataIC50: 0.0400nMAssay Description:Binding affinity against rat Opioid receptor delta 1 expressed in CHO cells determined by competitive inhibition of 1 nM [3H]DPDPE.More data for this Ligand-Target Pair
Affinity DataIC50: 0.0710nMAssay Description:Inhibitory activity against forskolin-stimulated cAMP production in chinese hamster ovary cells expressing Opioid receptor delta 1More data for this Ligand-Target Pair
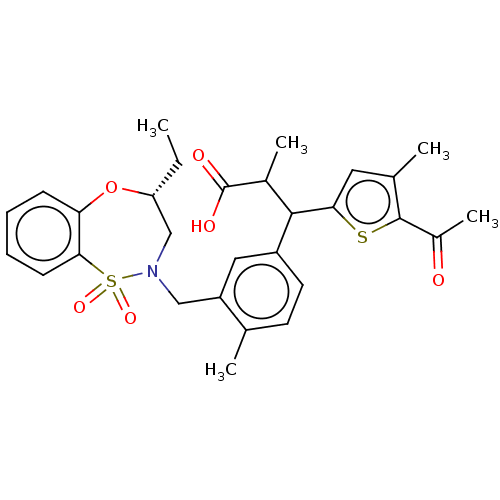
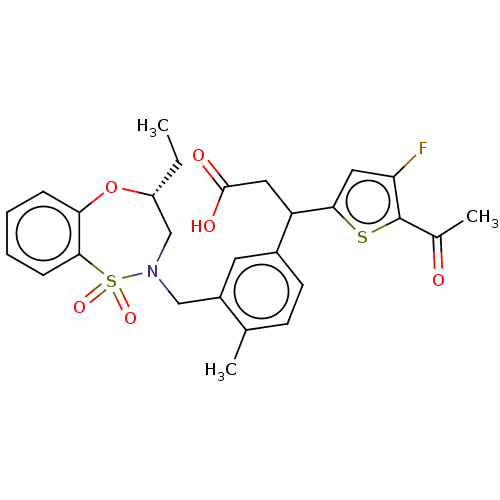
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.140nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
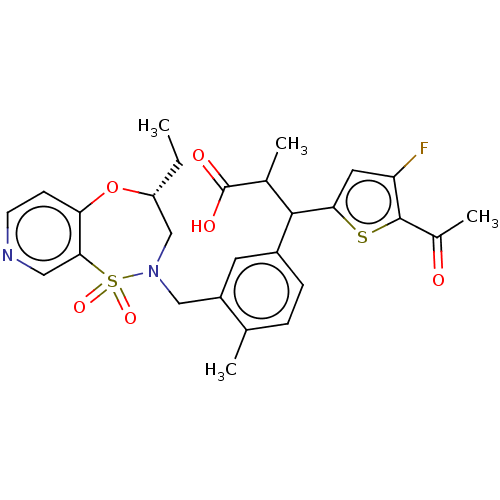
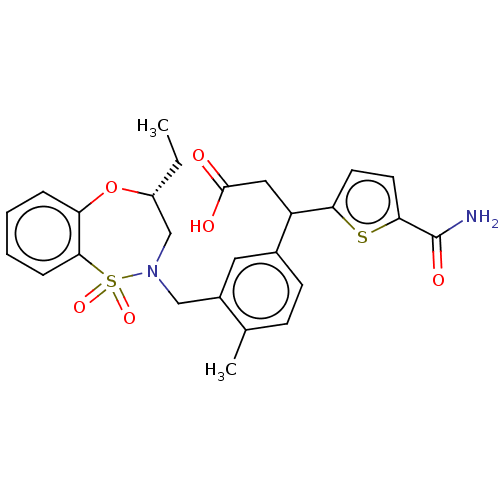
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.150nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
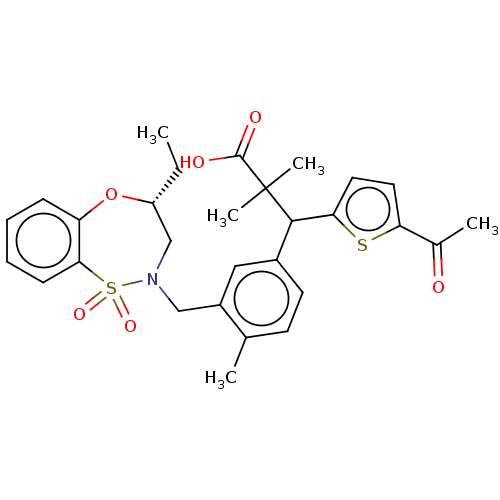
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.170nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
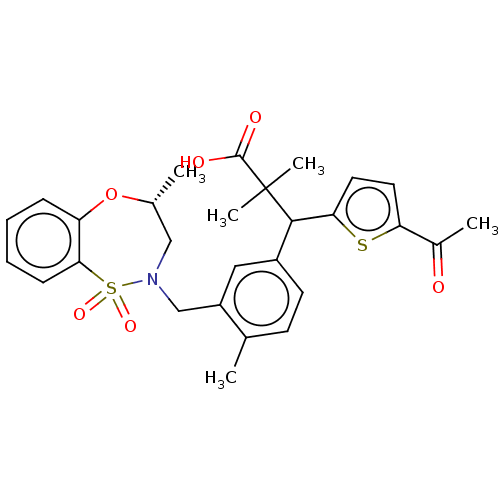
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.170nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.170nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.190nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.200nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of recombinant rat soluble epoxide hydrolase assessed as increase in fluorescence intensity by fluorescence plate readerMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of recombinant rat soluble epoxide hydrolase assessed as increase in fluorescence intensity by fluorescence plate readerMore data for this Ligand-Target Pair
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.230nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.230nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.240nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.25nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
Affinity DataIC50: 0.25nMAssay Description:Binding affinity against rat mu-opioid receptor expressed in CHO cells by competitive inhibition of 1 nM [3H]DAMGOMore data for this Ligand-Target Pair
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.270nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.270nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.280nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.300nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.300nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.310nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.360nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.360nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.390nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair
TargetKelch-like ECH-associated protein 1/Nuclear factor erythroid 2-related factor 2(Human)
Senju Pharmaceutical Co.
US Patent
Senju Pharmaceutical Co.
US Patent
Affinity DataIC50: 0.390nMAssay Description:The inhibitory activity of each compound on the binding between NRF2 and KEAP1 was measured by bioluminescence resonance energy transfer (BRET) assay...More data for this Ligand-Target Pair