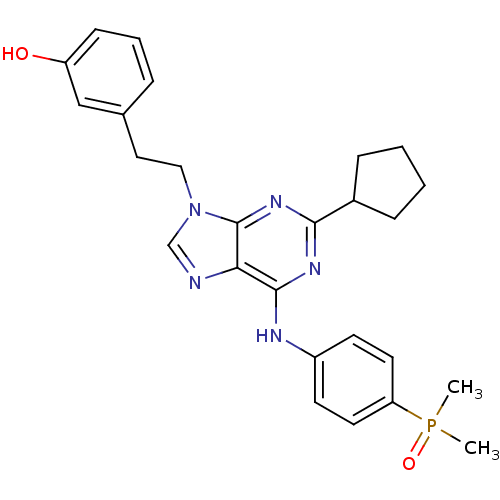
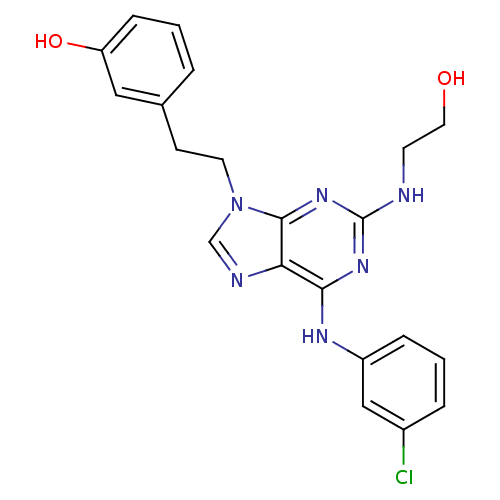
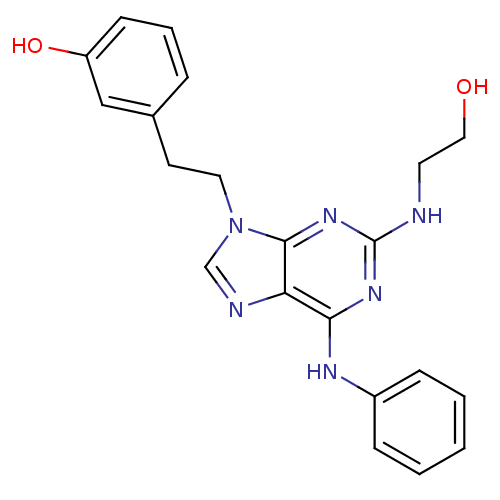
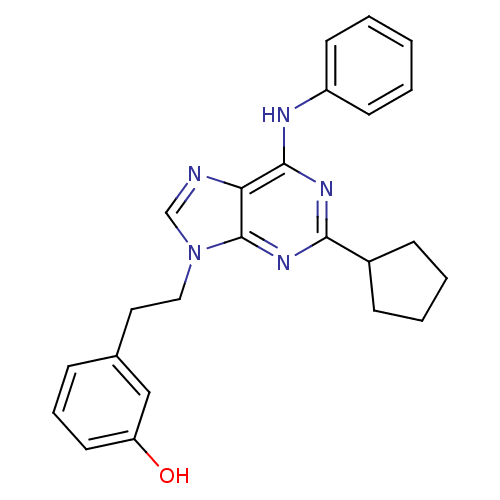
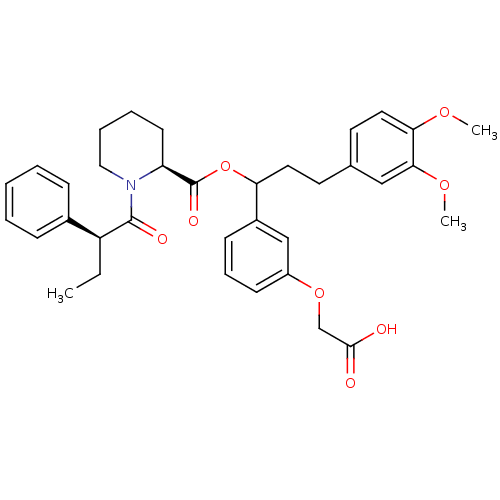
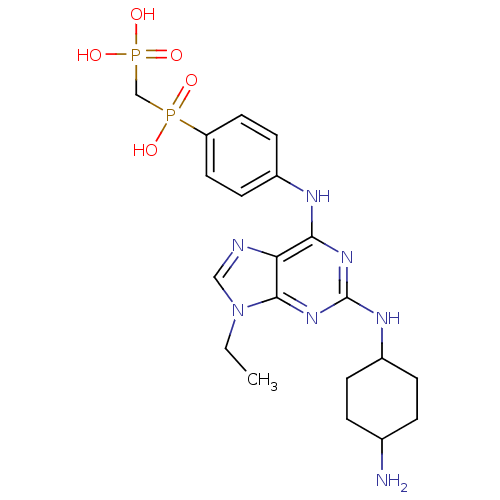
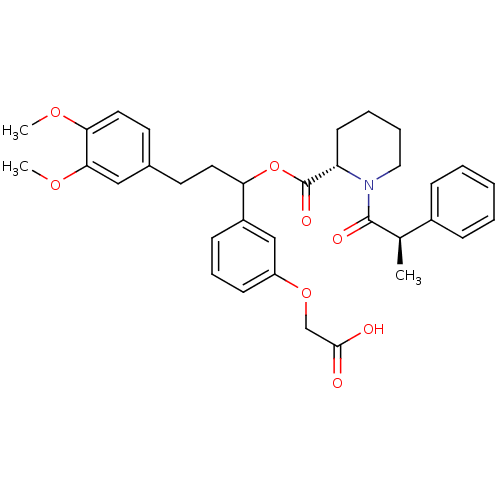
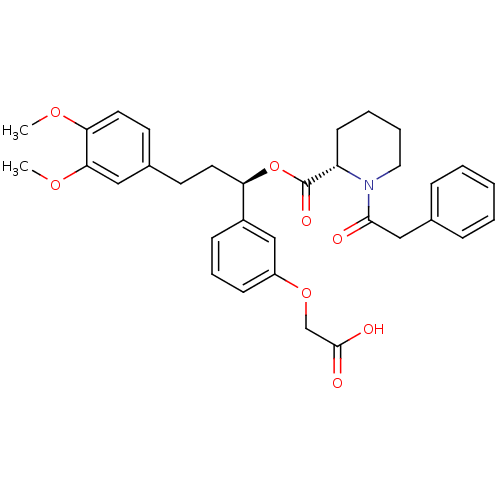
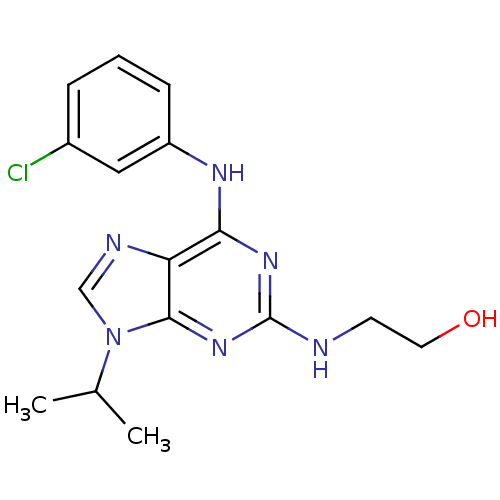
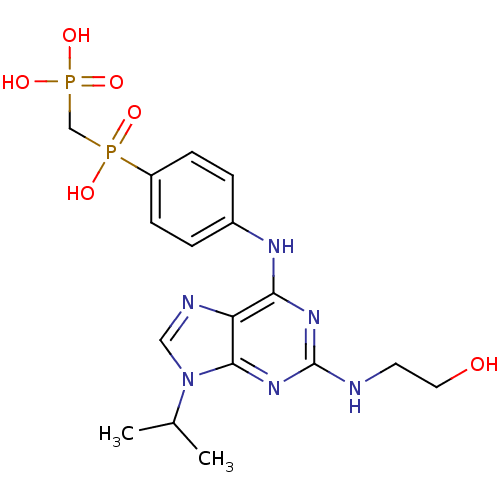
Affinity DataIC50: 0.450nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 3.80nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 4.40nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 5.80nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
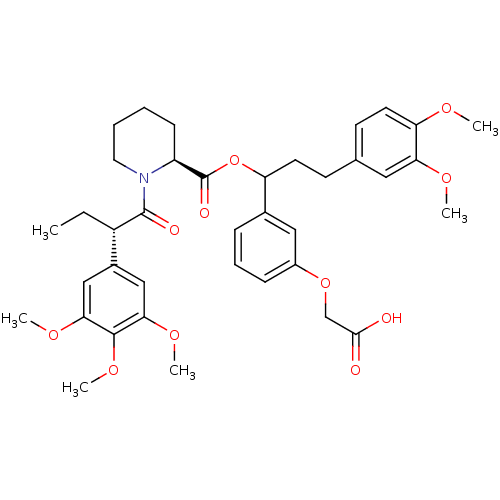
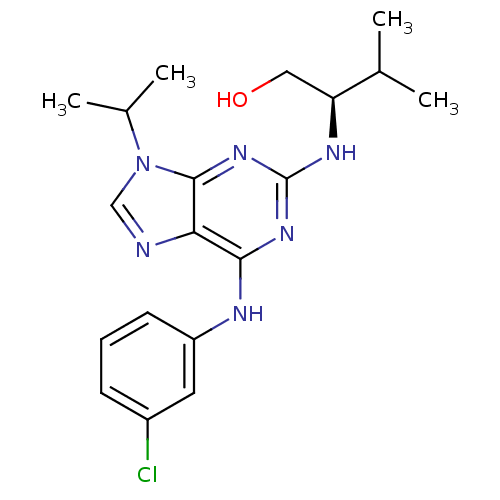
Affinity DataIC50: 25nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
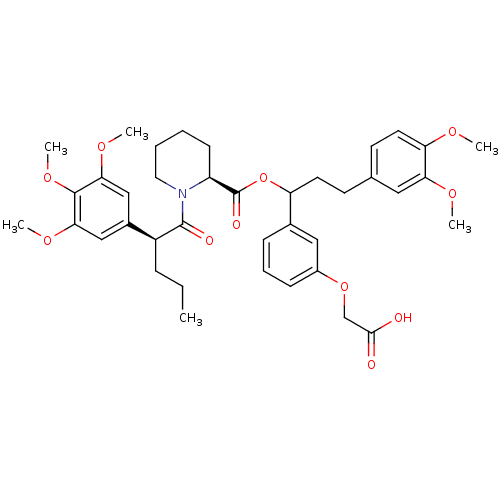
Affinity DataIC50: 26nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
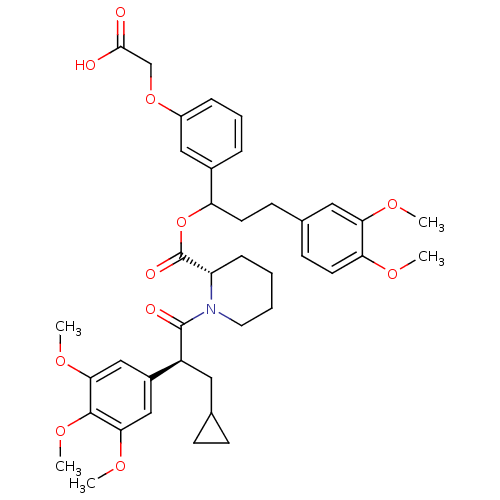
Affinity DataIC50: 34nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 43nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 65nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 66nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
Affinity DataIC50: 67nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 70nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 94nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 126nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 133nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 187nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
Affinity DataIC50: 239nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 240nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 273nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 590nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Ariad Gene Therapeutics
Curated by ChEMBL
Ariad Gene Therapeutics
Curated by ChEMBL
Affinity DataIC50: 1.53E+3nMAssay Description:Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization.More data for this Ligand-Target Pair
Affinity DataIC50: 1.59E+3nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 2.01E+3nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 2.11E+3nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
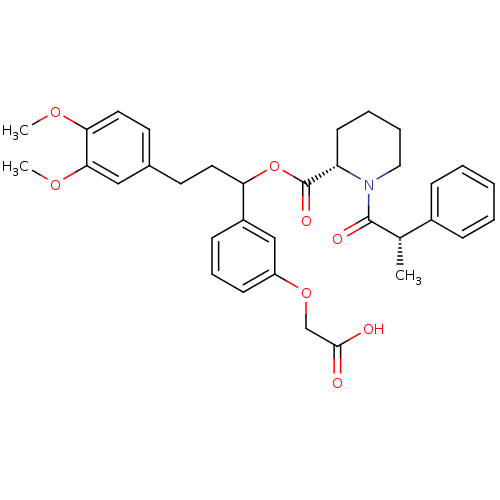
TargetSodium- and chloride-dependent GABA transporter 1(Homo sapiens (Human))
Drexel University College Of Medicine
Curated by ChEMBL
Drexel University College Of Medicine
Curated by ChEMBL
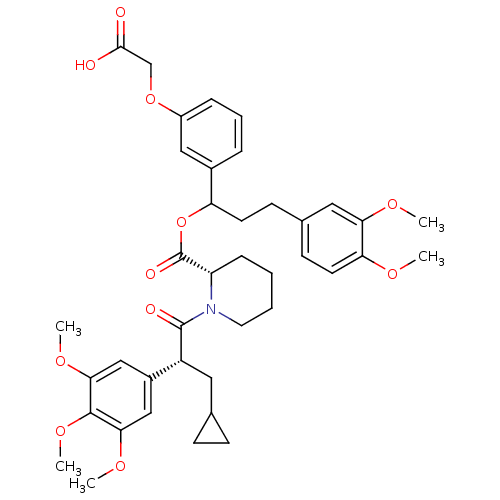
Affinity DataIC50: 2.06E+4nMAssay Description:Inhibition of GAT-1 (unknown origin) expressed in african green monkey COS7 cells using [3H]-GABA as substrate after 10 mins by scintillation countin...More data for this Ligand-Target Pair
Affinity DataIC50: 2.09E+4nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetExcitatory amino acid transporter 2(Homo sapiens (Human))
Drexel University College Of Medicine
Curated by ChEMBL
Drexel University College Of Medicine
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of EAAT2 (unknown origin) expressed in african green monkey COS7 cells using [3H]-glutamic acid as substrate after 10 mins by scintillatio...More data for this Ligand-Target Pair
TargetSodium- and chloride-dependent glycine transporter 1(Homo sapiens (Human))
Drexel University College Of Medicine
Curated by ChEMBL
Drexel University College Of Medicine
Curated by ChEMBL
Affinity DataIC50: >5.00E+5nMAssay Description:Inhibition of GlyT1 (unknown origin) expressed in MDCK cells using [3H]-glycine as substrate after 10 mins by scintillation countingMore data for this Ligand-Target Pair
TargetExcitatory amino acid transporter 3(Homo sapiens (Human))
Drexel University College Of Medicine
Curated by ChEMBL
Drexel University College Of Medicine
Curated by ChEMBL
Affinity DataIC50: >5.00E+5nMAssay Description:Inhibition of EAAT3 (unknown origin) expressed in african green monkey COS7 cells using [3H]-glutamic acid as substrate after 10 mins by scintillatio...More data for this Ligand-Target Pair
TargetExcitatory amino acid transporter 1(Homo sapiens (Human))
Drexel University College Of Medicine
Curated by ChEMBL
Drexel University College Of Medicine
Curated by ChEMBL
Affinity DataIC50: >5.00E+5nMAssay Description:Inhibition of EAAT1 (unknown origin) expressed in african green monkey COS7 cells using [3H]-glutamic acid as substrate after 10 mins by scintillatio...More data for this Ligand-Target Pair
TargetSodium- and chloride-dependent GABA transporter 3(Homo sapiens (Human))
Drexel University College Of Medicine
Curated by ChEMBL
Drexel University College Of Medicine
Curated by ChEMBL
Affinity DataIC50: >5.00E+5nMAssay Description:Inhibition of GAT-3 (unknown origin) expressed in african green monkey COS7 cells using [3H]-GABA as substrate after 10 mins by scintillation countin...More data for this Ligand-Target Pair