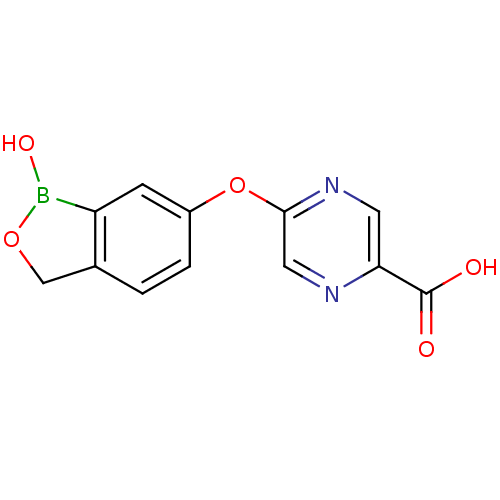
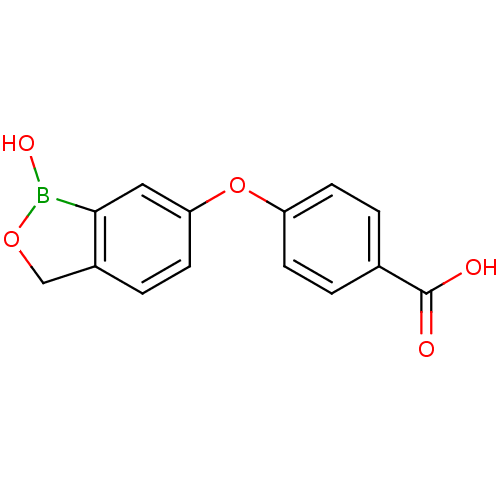
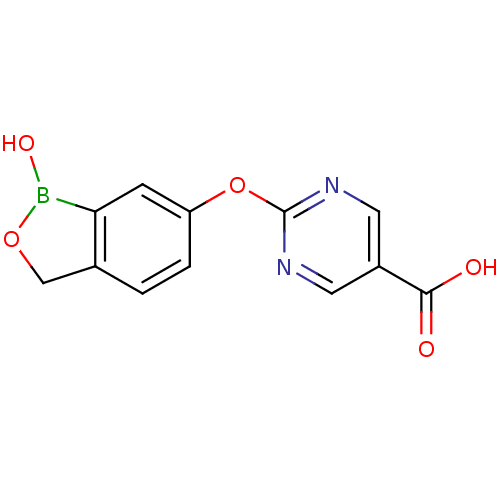
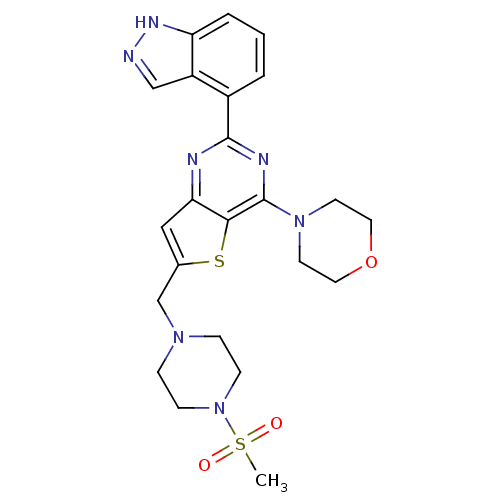
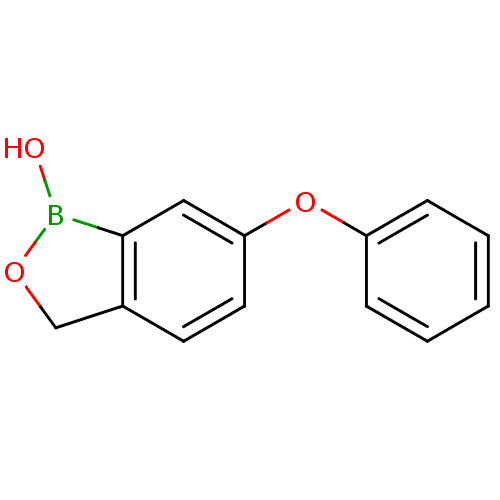
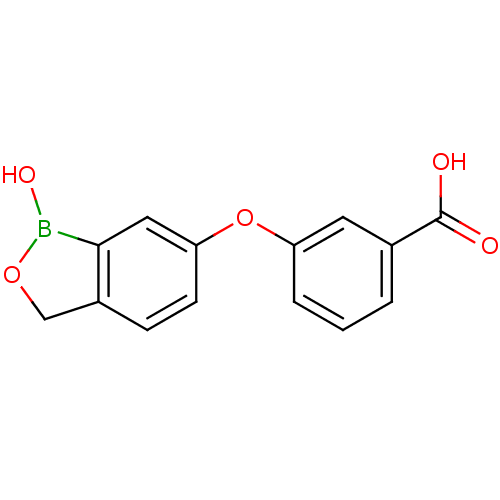
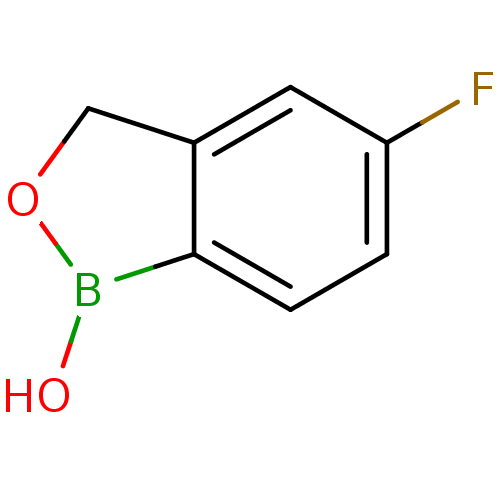
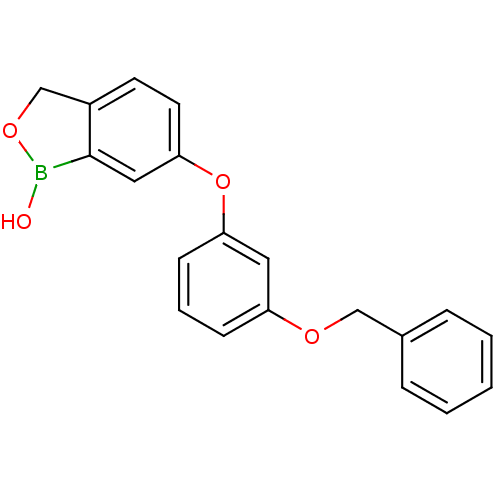
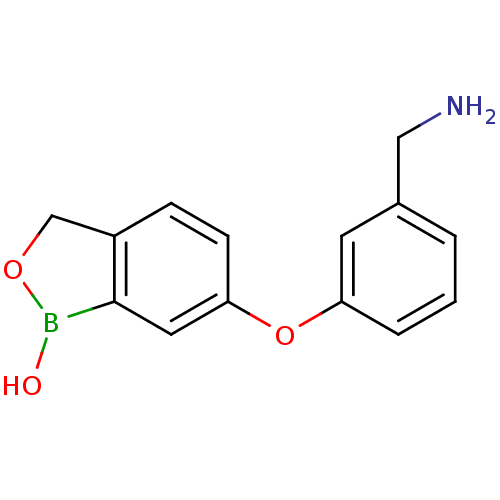
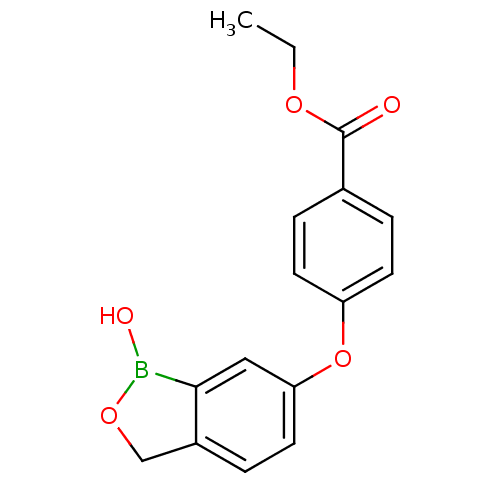
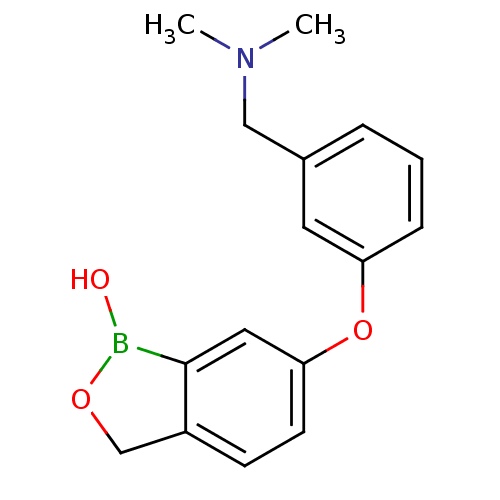
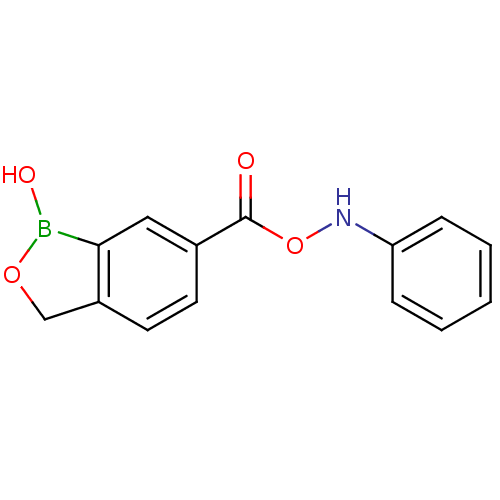
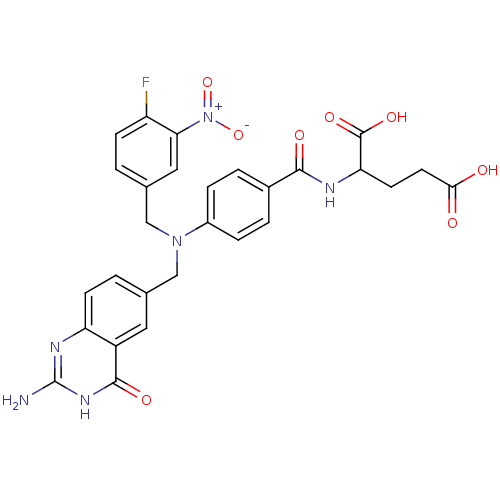
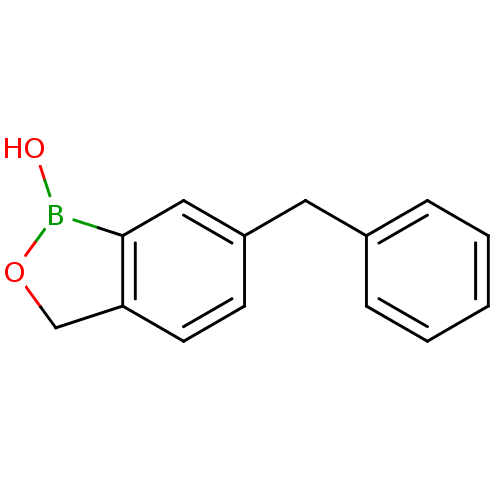
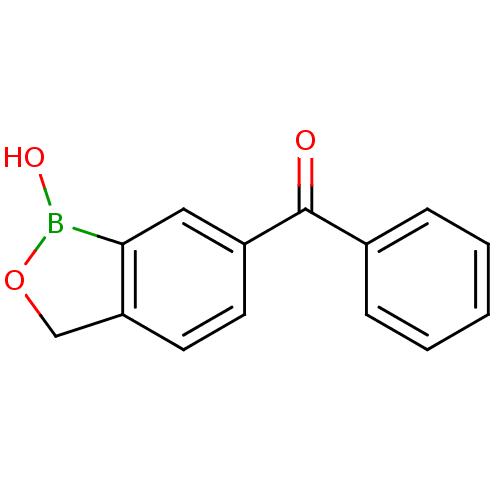
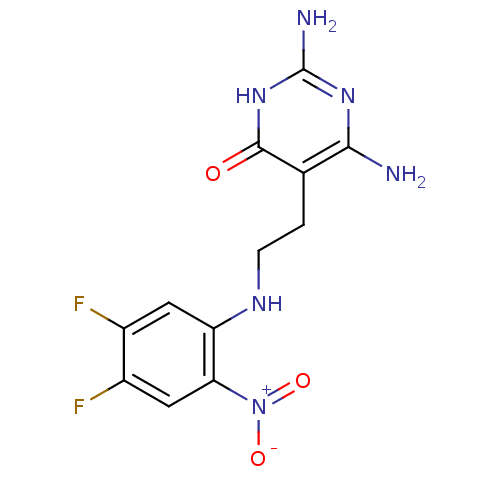
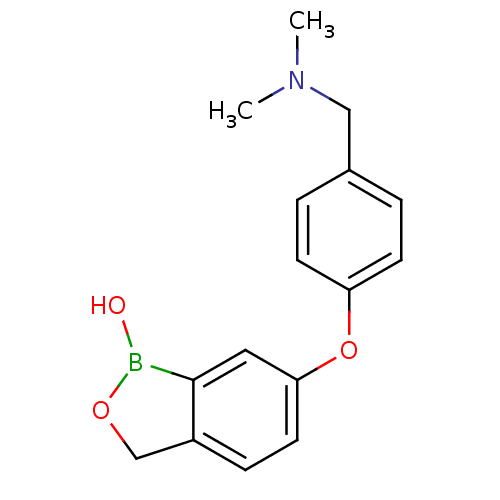
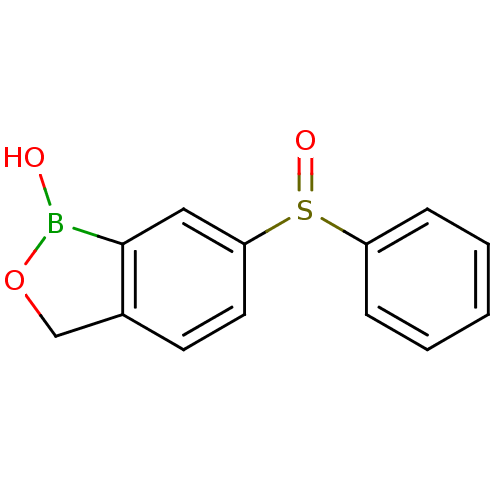
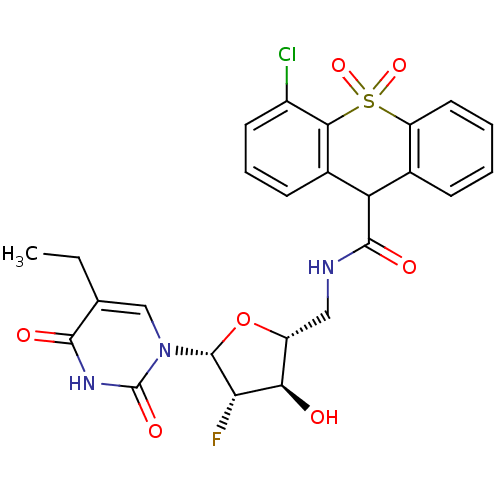
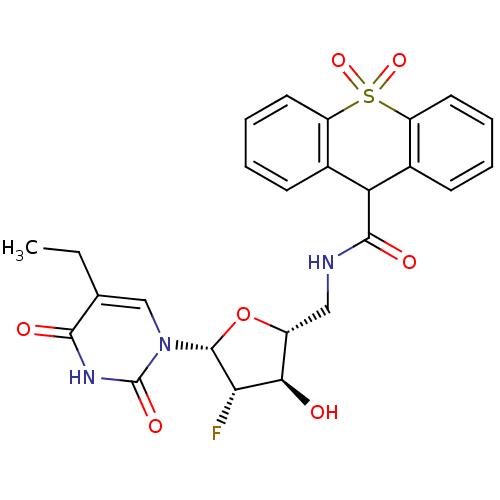
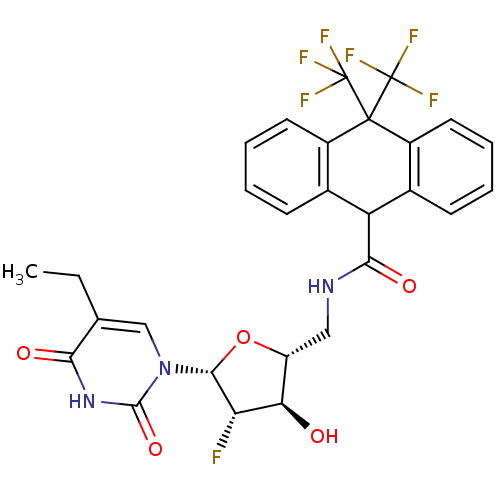
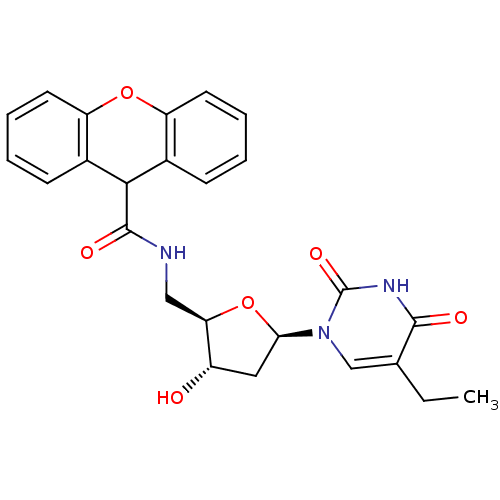
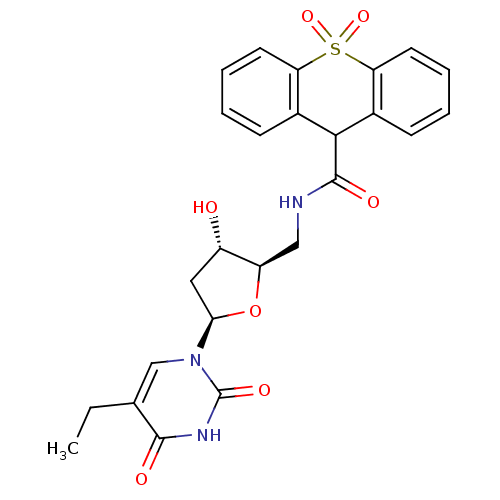
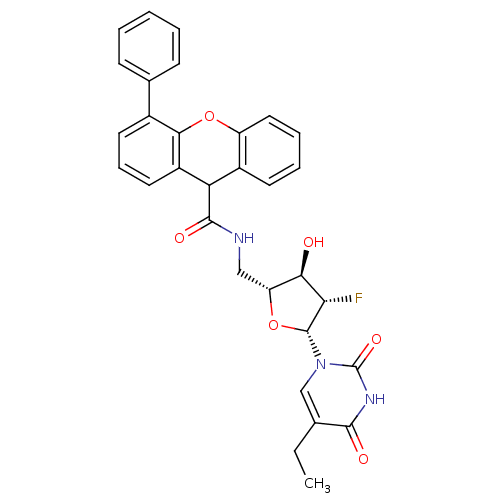
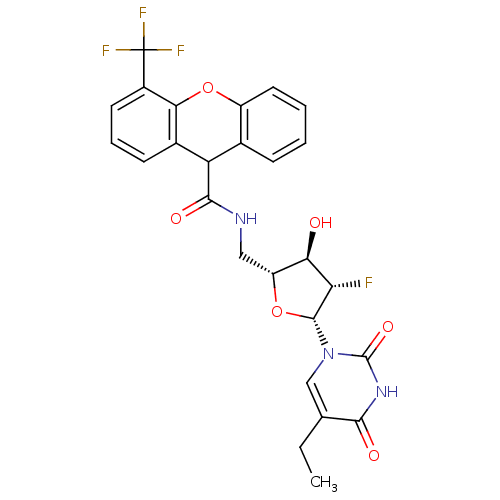
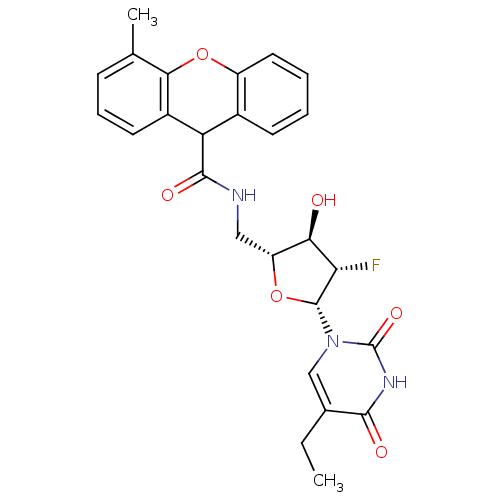
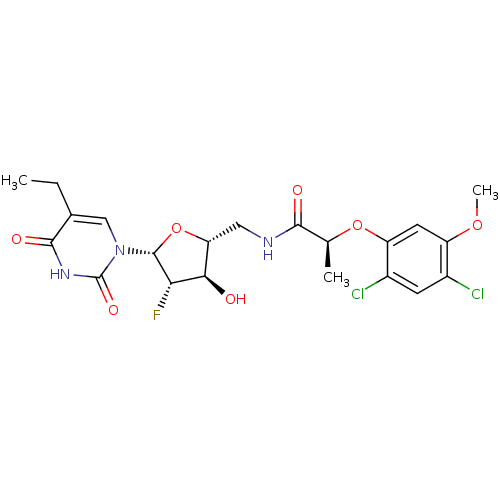
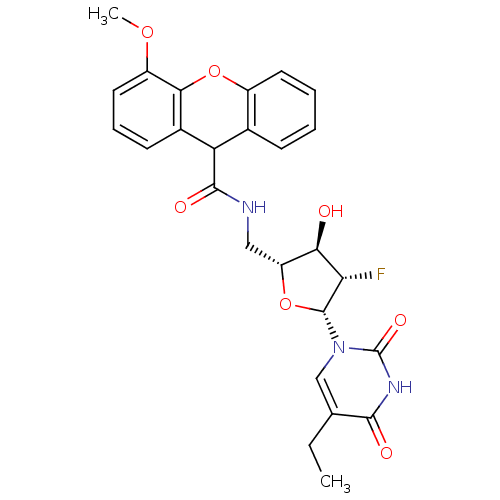
Affinity DataKi: 20nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 580nM ΔG°: -35.6kJ/molepH: 7.5 T: 2°CAssay Description:Mammalian target of rapamycin (mTOR) was assayed by monitoring phosphorylation of GFP-4EBP using a homogeneous time-resolved fluorescence resonance e...More data for this Ligand-Target Pair
Affinity DataKi: 710nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.35E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.44E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetLeucine--tRNA ligase, cytoplasmic(Saccharomyces cerevisiae S288c)
Anacor Pharmaceuticals
Curated by ChEMBL
Anacor Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 1.85E+3nMAssay Description:Inhibition of Saccharomyces cerevisiae cytoplasmic leucyl-tRNA synthetase after 20 minsMore data for this Ligand-Target Pair
Affinity DataKi: 2.27E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.75E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.33E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 4.73E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5.55E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 6.00E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 7.28E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 7.72E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 8.40E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 8.73E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 9.09E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.33E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetBifunctional purine biosynthesis protein ATIC(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 1.70E+4nMAssay Description:In vitro inhibitory activity against Aminoimidazole carboxamide ribonucleotide transformylaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.98E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.85E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetLeucine--tRNA ligase, cytoplasmic(Saccharomyces cerevisiae S288c)
Anacor Pharmaceuticals
Curated by ChEMBL
Anacor Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 3.14E+4nMAssay Description:Inhibition of Saccharomyces cerevisiae cytoplasmic leucyl-tRNA synthetase after 2 minsMore data for this Ligand-Target Pair
TargetTrifunctional purine biosynthetic protein adenosine-3(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 3.50E+4nMAssay Description:In vitro inhibitory activity against Glycinamide ribonucleotide transformylase (GAR Tfase) after 3 min at 250 uMMore data for this Ligand-Target Pair
Affinity DataKi: 4.06E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetTrifunctional purine biosynthetic protein adenosine-3(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 4.50E+4nMAssay Description:In vitro inhibitory activity against Glycinamide ribonucleotide transformylase (GAR Tfase)More data for this Ligand-Target Pair
Affinity DataKi: 4.66E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetBifunctional purine biosynthesis protein ATIC(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 5.20E+4nMAssay Description:In vitro inhibitory activity against Aminoimidazole carboxamide ribonucleotide transformylaseMore data for this Ligand-Target Pair
Affinity DataKi: 5.80E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 6.32E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetTrifunctional purine biosynthetic protein adenosine-3(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:In vitro inhibitory activity against Glycinamide ribonucleotide transformylase (GAR Tfase)More data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMAssay Description:Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK)More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK)More data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMAssay Description:Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK)More data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMAssay Description:Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK)More data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMAssay Description:Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK)More data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK)More data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK)More data for this Ligand-Target Pair
Affinity DataIC50: 0.140nMAssay Description:Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK)More data for this Ligand-Target Pair
Affinity DataIC50: 0.140nMAssay Description:Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK)More data for this Ligand-Target Pair
Affinity DataIC50: 0.140nMAssay Description:Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK)More data for this Ligand-Target Pair
Affinity DataIC50: 0.190nMAssay Description:Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK)More data for this Ligand-Target Pair
Affinity DataIC50: 0.190nMAssay Description:Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK)More data for this Ligand-Target Pair
TargetThymidine kinase(Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...)
Roche Discovery Welwyn
Curated by ChEMBL
Roche Discovery Welwyn
Curated by ChEMBL
Affinity DataIC50: 0.190nMAssay Description:Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK)More data for this Ligand-Target Pair
Affinity DataIC50: 0.210nMAssay Description:Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK)More data for this Ligand-Target Pair
Affinity DataIC50: 0.210nMAssay Description:Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK)More data for this Ligand-Target Pair
TargetThymidine kinase(Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...)
Roche Discovery Welwyn
Curated by ChEMBL
Roche Discovery Welwyn
Curated by ChEMBL
Affinity DataIC50: 0.270nMAssay Description:Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK)More data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMAssay Description:Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK)More data for this Ligand-Target Pair