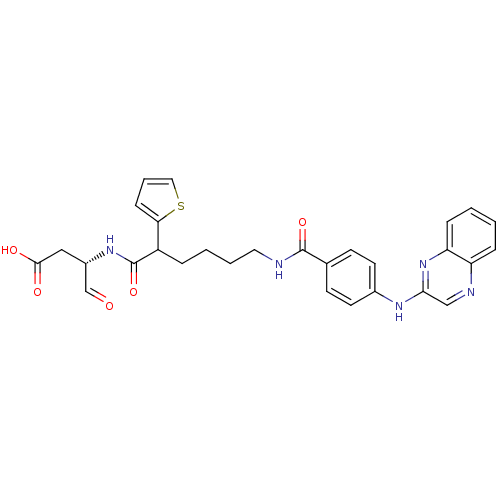
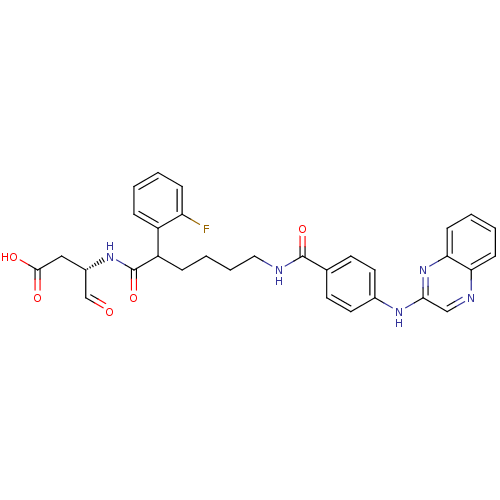
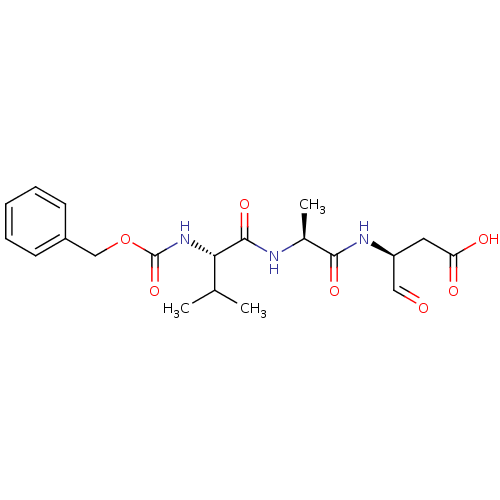
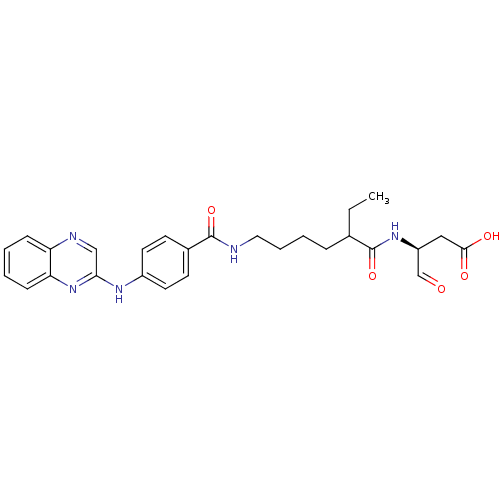
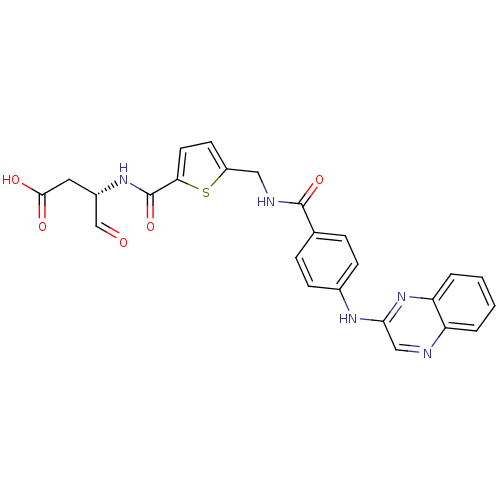
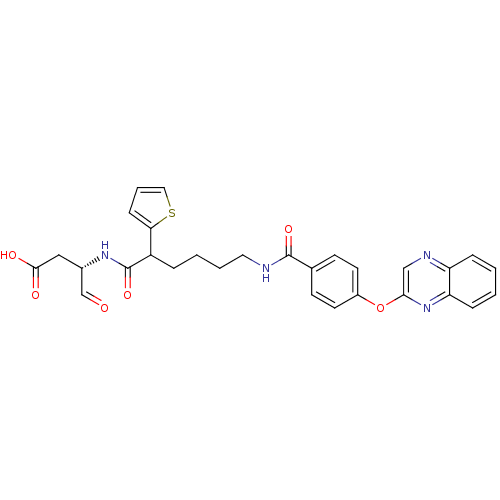
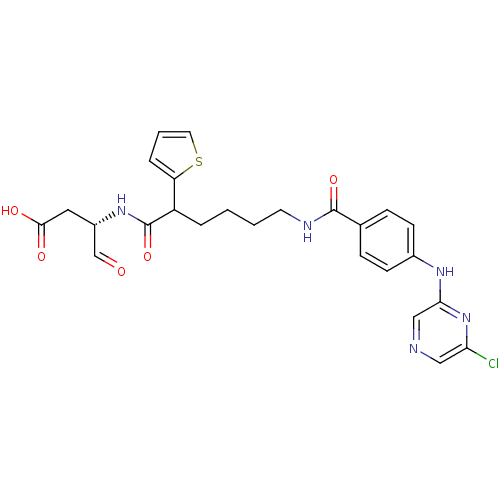
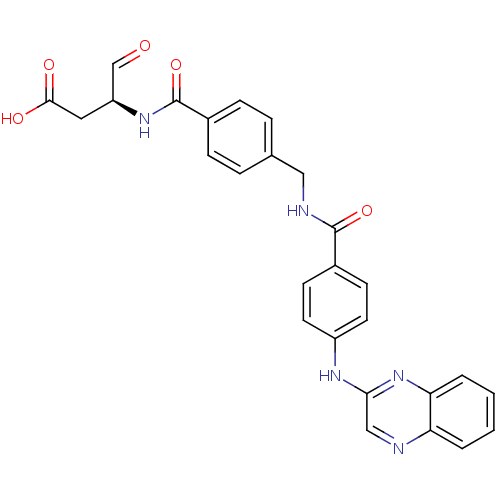
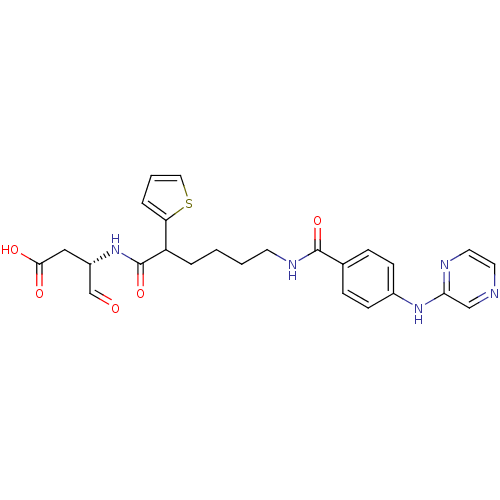
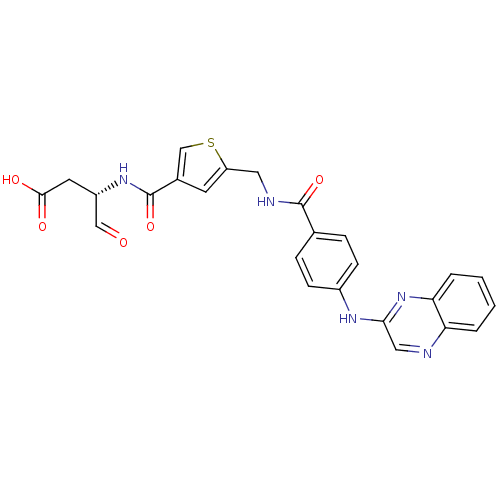
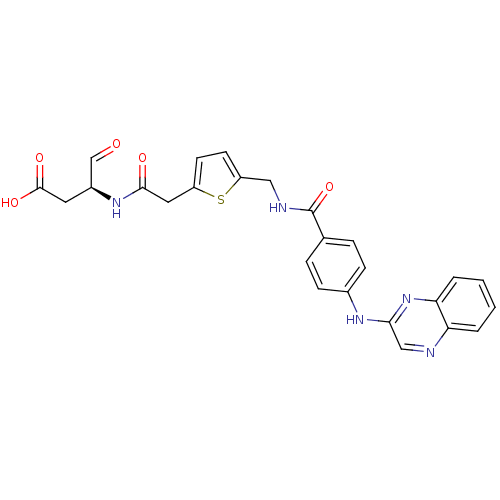
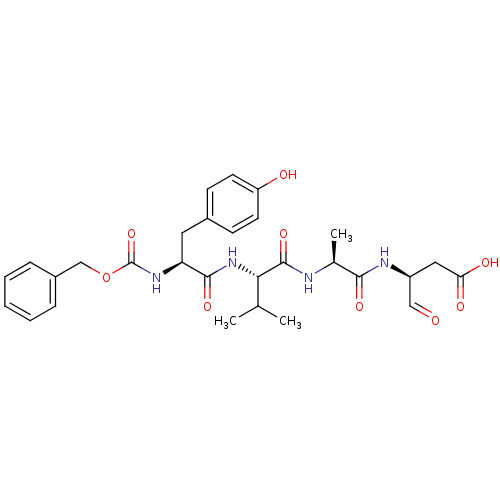
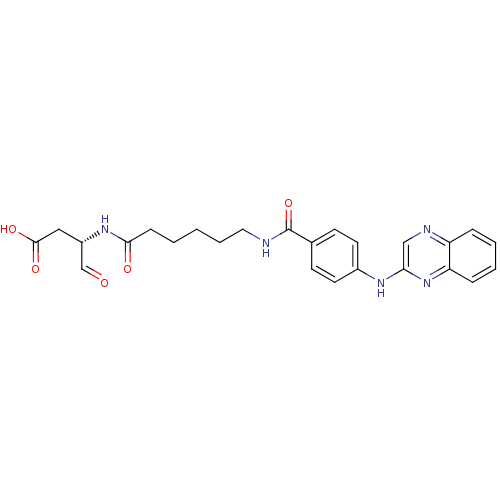
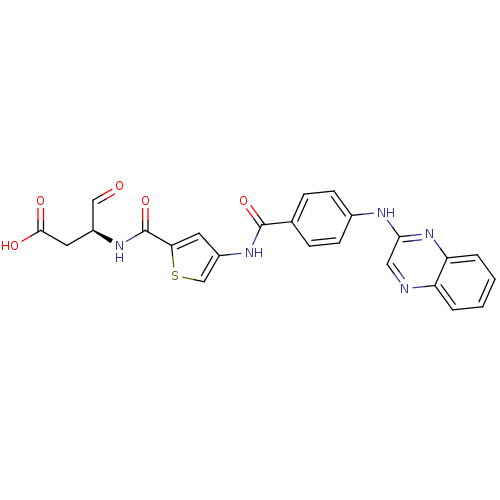
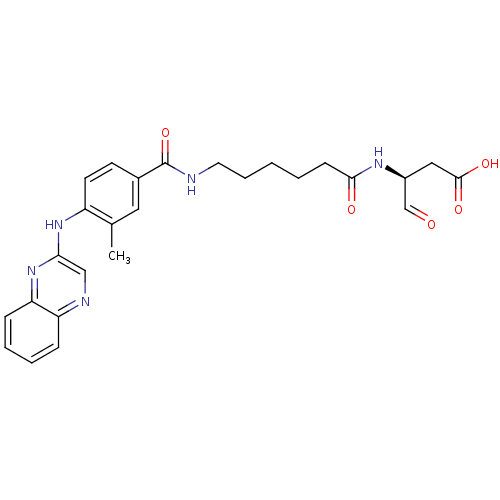
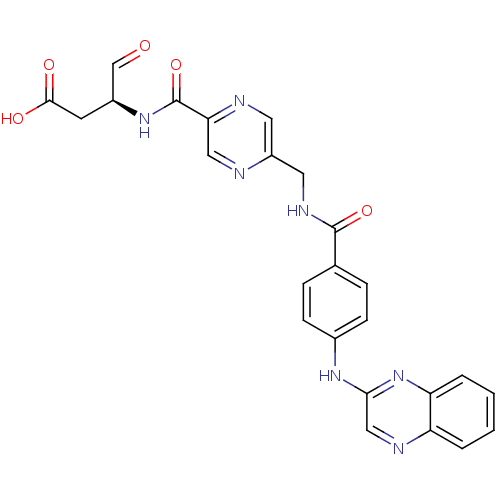
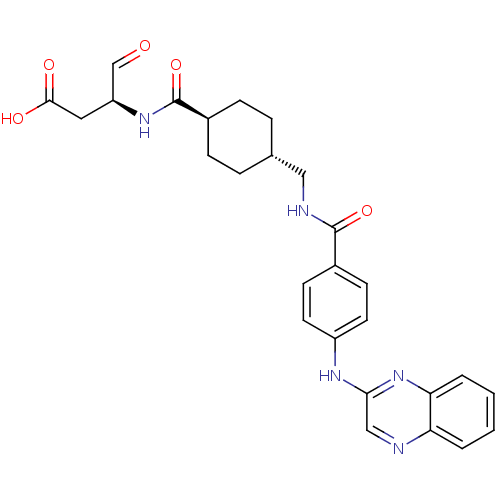
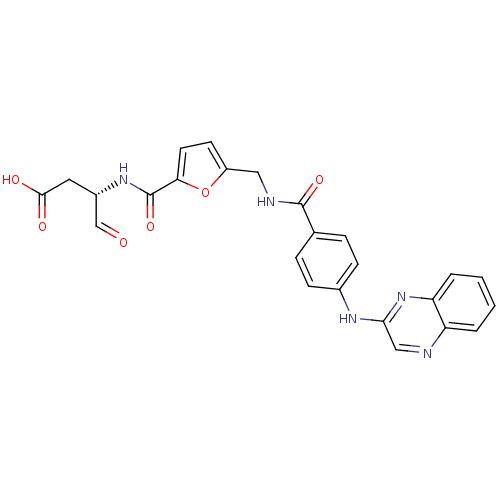
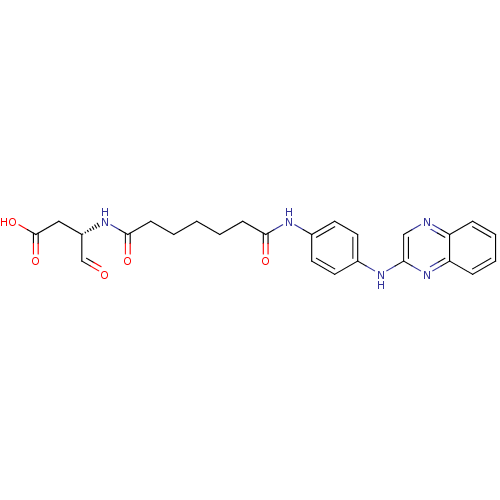
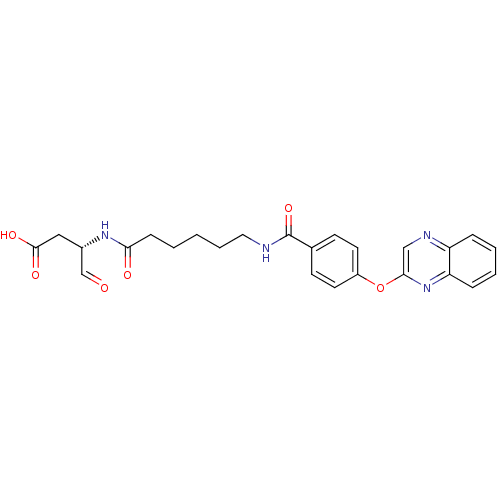
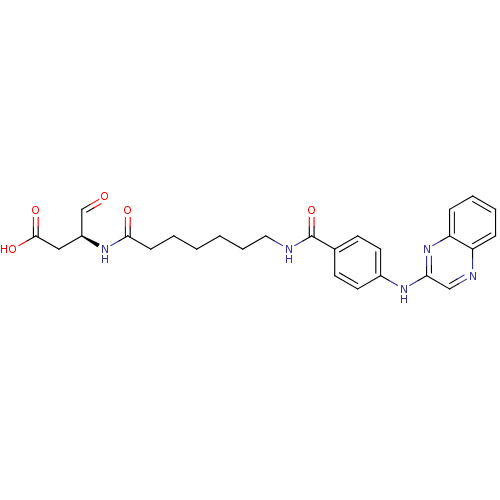
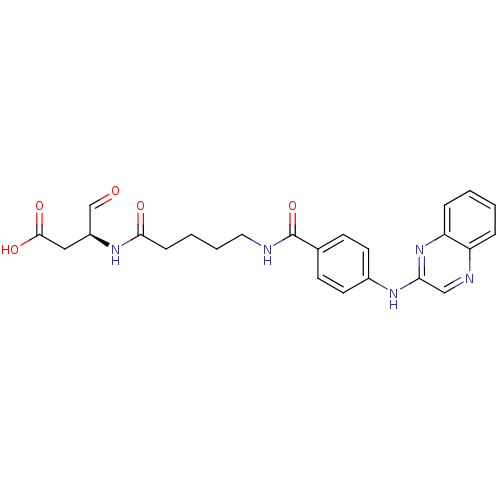
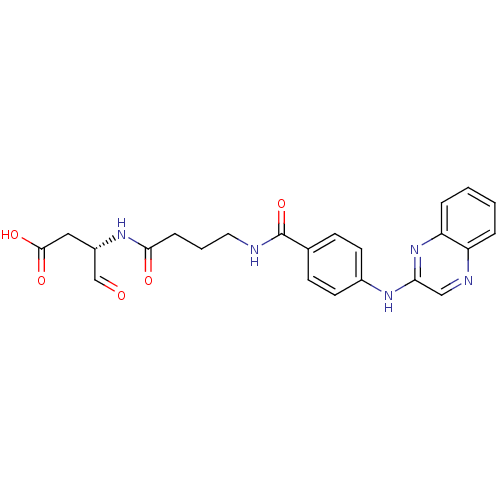
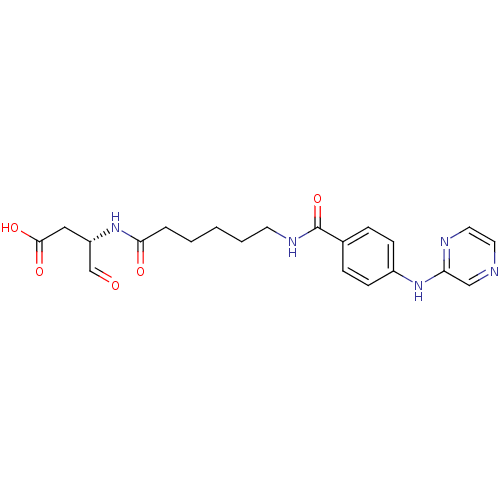
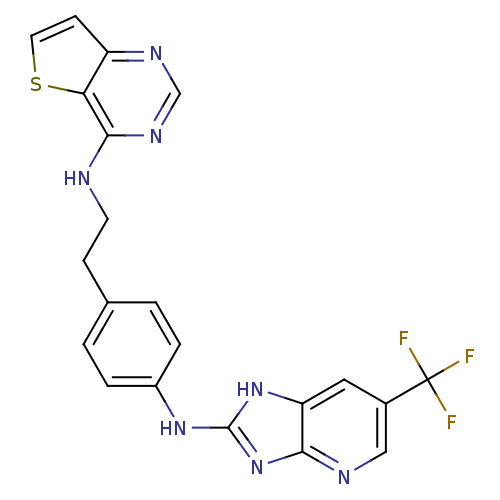
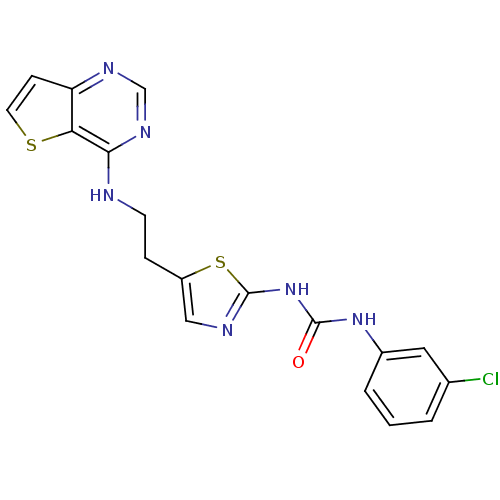
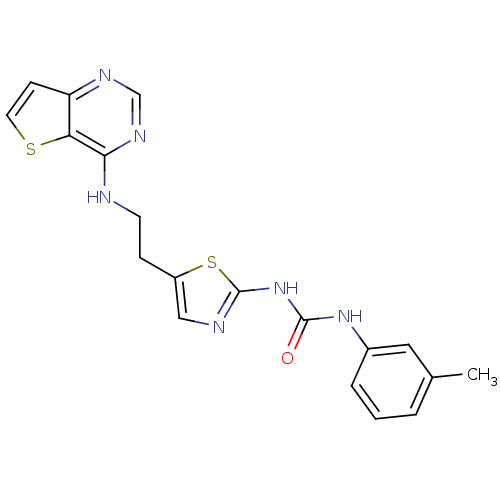
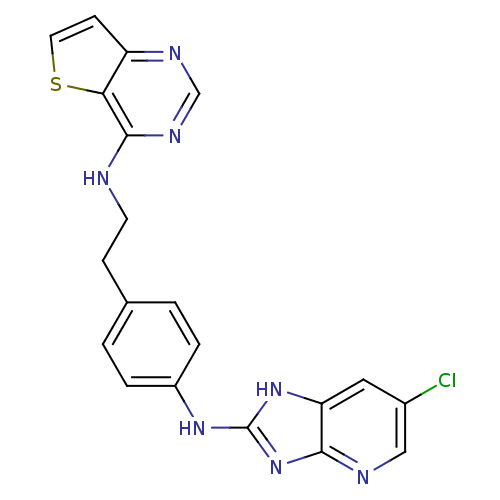
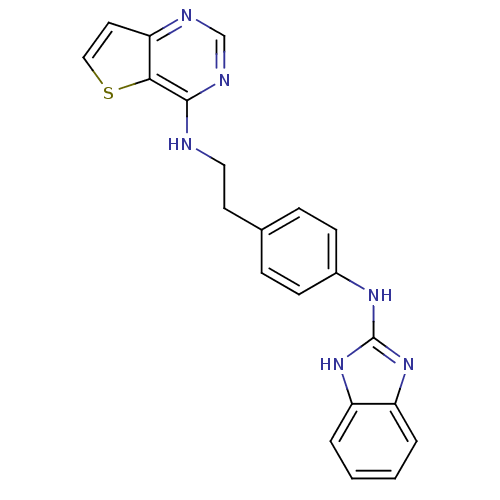
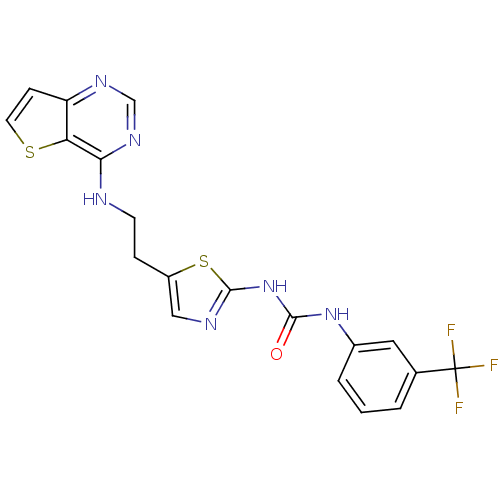
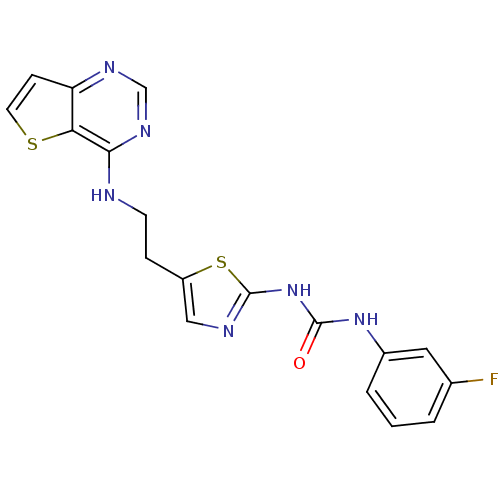
Affinity DataKi: 4nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 35nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 54nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 120nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 120nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 180nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 190nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 260nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 320nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 1.95E+3nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 2.10E+3nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 2.10E+3nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 2.40E+3nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 2.60E+3nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+3nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 5.20E+3nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataKi: 3.70E+4nMAssay Description:Inhibitory activity against human Caspase 1More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.2 T: 2°CAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of Aurora B after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of Aurora B after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of Aurora B after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMpH: 7.2 T: 2°CAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMpH: 7.2 T: 2°CAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of Aurora A after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of Aurora B after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of Aurora B after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMpH: 7.2 T: 2°CAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of Aurora B after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of Aurora A after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of Aurora A after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of Aurora B after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of Aurora A after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMpH: 7.2 T: 2°CAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair

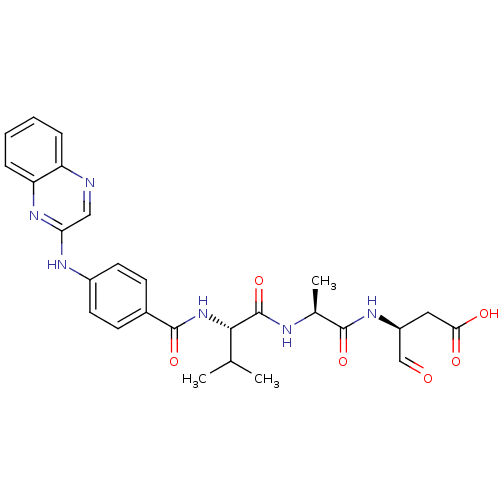
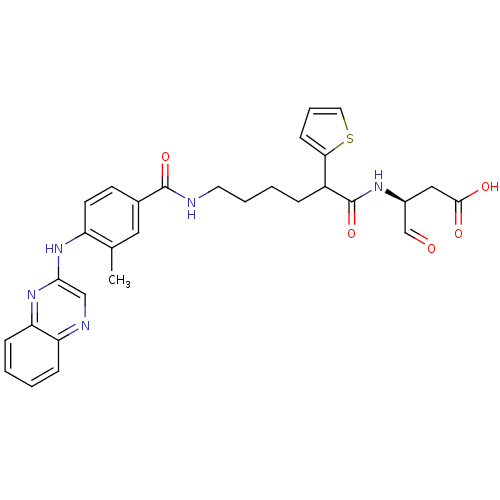
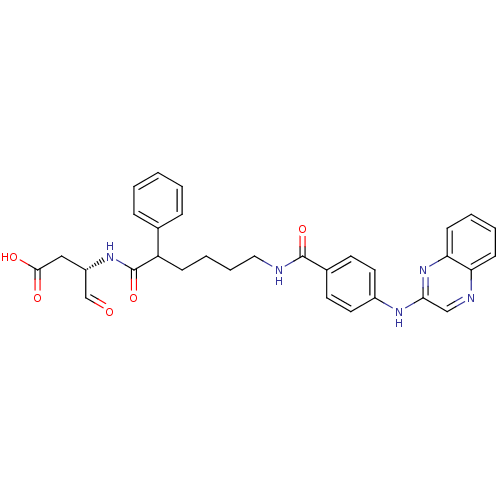
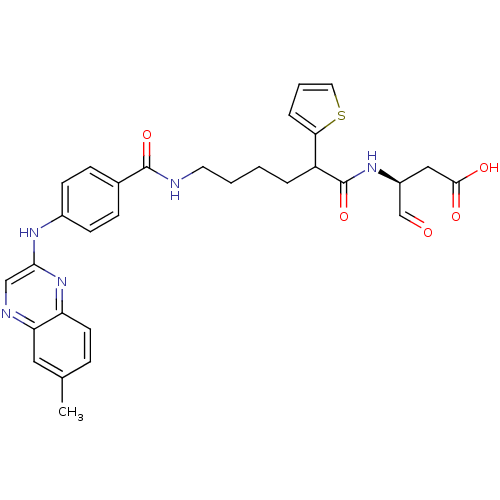
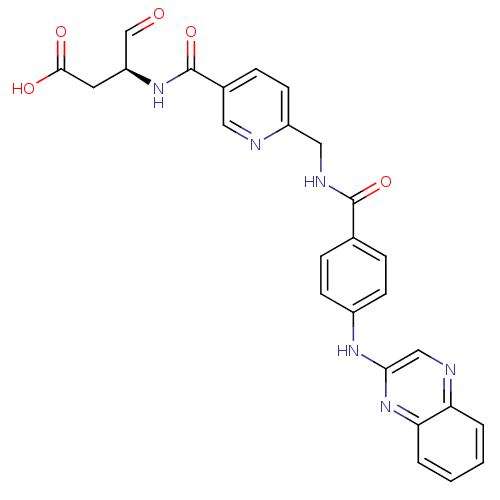
 3D Structure (crystal)
3D Structure (crystal)