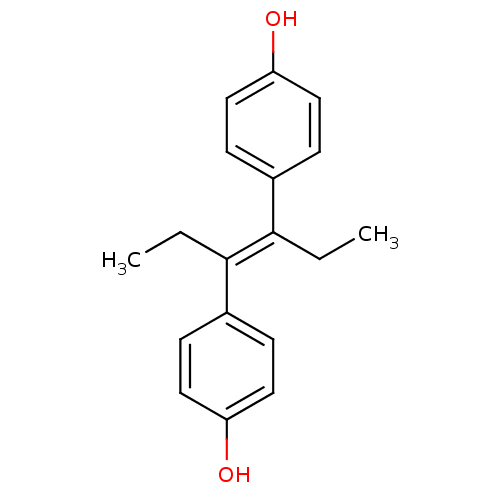
Affinity DataIC50: 34nMAssay Description:Inhibition of human platelets COX1 assessed as PGE2 production using arachidonic acid as substrate preincubated for 15 mins measured after 15 mins by...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
Rwth Aachen University
Curated by ChEMBL
Rwth Aachen University
Curated by ChEMBL
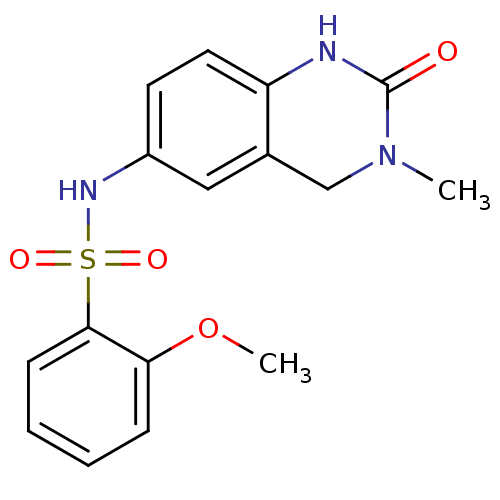
Affinity DataIC50: 35nMAssay Description:Inhibition of recombinant AKR1C3 (unknown origin) using S-tetralol as substrateMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
Rwth Aachen University
Curated by ChEMBL
Rwth Aachen University
Curated by ChEMBL
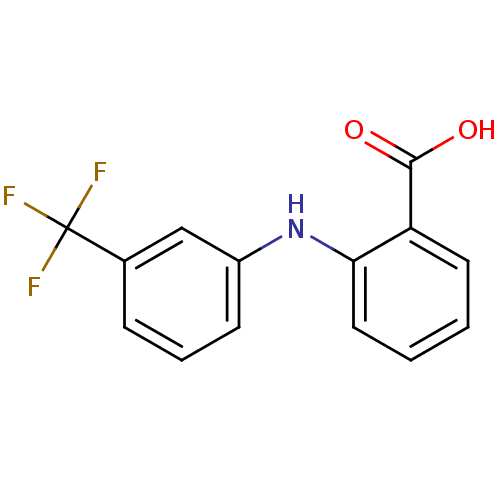
Affinity DataIC50: 36nMAssay Description:Inhibition of recombinant AKR1C3 (unknown origin) using S-tetralol as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:Inhibition of human platelets COX1 assessed as PGE2 production using arachidonic acid as substrate preincubated for 15 mins measured after 15 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:Inhibition of human platelet COX1 using arachidonic acid as substrate preincubated for 15 mins before arachidonic acid addition measured after 15 min...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
Rwth Aachen University
Curated by ChEMBL
Rwth Aachen University
Curated by ChEMBL
Affinity DataIC50: 57nMAssay Description:Inhibition of recombinant AKR1C3 (unknown origin) using S-tetralol as substrateMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
Rwth Aachen University
Curated by ChEMBL
Rwth Aachen University
Curated by ChEMBL
Affinity DataIC50: 86nMAssay Description:Inhibition of recombinant AKR1C3 (unknown origin) using S-tetralol as substrateMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
Rwth Aachen University
Curated by ChEMBL
Rwth Aachen University
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Inhibition of recombinant AKR1C2 (unknown origin) using S-tetralol as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 156nMAssay Description:Inhibition of human recombinant COX2 expressed in Sf21 cells assessed as PGE2 production using arachidonic acid as substrate preincubated for 15 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 156nMAssay Description:Inhibition of human recombinant COX2 expressed in Sf21 cells assessed as PGE2 production using arachidonic acid as substrate preincubated for 15 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 170nMAssay Description:Inhibition of human recombinant COX2 expressed in Sf21 cells using arachidonic acid as substrate preincubated for 15 mins before arachidonic acid add...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
Rwth Aachen University
Curated by ChEMBL
Rwth Aachen University
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:Inhibition of recombinant AKR1C2 (unknown origin) using S-tetralol as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Inhibition of BRD4 in human HEL cells harboring JAK2 V617F mutant assessed as decrease in metabolic activity measured after 72 hrs by MTT assayMore data for this Ligand-Target Pair
Affinity DataIC50: 610nMAssay Description:Displacement of [3H]-estradiol from human recombinant ERbeta expressed in Sf21 cells after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 770nMAssay Description:Displacement of [3H]-estradiol from human recombinant ERalpha expressed in Sf21 cells after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of BRD4 in human MOLM-14 cells harboring FLT3-ITD mutant assessed as decrease in metabolic activity measured after 72 hrs by MTT assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of COX1 (unknown origin) using arachidonic acid substrate by colorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of BRD4 in human HEL cells harboring JAK2 V617F mutant assessed as decrease in metabolic activity measured after 72 hrs by MTT assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of BRD4 in human MOLM-14 cells harboring FLT3-ITD mutant assessed as decrease in metabolic activity measured after 72 hrs by MTT assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of COX2 (unknown origin) using arachidonic acid substrate by colorimetric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
Rwth Aachen University
Curated by ChEMBL
Rwth Aachen University
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibition of recombinant AKR1C2 (unknown origin) using S-tetralol as substrateMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
Rwth Aachen University
Curated by ChEMBL
Rwth Aachen University
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of recombinant AKR1C2 (unknown origin) using S-tetralol as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+4nMAssay Description:Inhibition of COX1 (unknown origin) using arachidonic acid substrate by colorimetric assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Rwth Aachen University
Curated by ChEMBL
Rwth Aachen University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human ERG expressed in HEK293 cells assessed as inhibition of tail current at holding potential of -70 mV after 10 mins by whole-cell p...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of COX2 (unknown origin) using arachidonic acid substrate by colorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of COX1 (unknown origin) using arachidonic acid substrate by colorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of COX2 (unknown origin) using arachidonic acid substrate by colorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of COX2 (unknown origin) using arachidonic acid substrate by colorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of COX1 (unknown origin) using arachidonic acid substrate by colorimetric assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Rwth Aachen University
Curated by ChEMBL
Rwth Aachen University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human ERG expressed in HEK293 cells assessed as inhibition of tail current at holding potential of -70 mV after 10 mins by whole-cell p...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of COX1More data for this Ligand-Target Pair
Affinity DataEC50: 2.60E+6nMAssay Description:Binding affinity to rat bile acid-sensitive ion channel expressed in xenopus oocytesMore data for this Ligand-Target Pair
Affinity DataEC50: 2.90E+6nMAssay Description:Binding affinity to rat bile acid-sensitive ion channel expressed in xenopus oocytesMore data for this Ligand-Target Pair
Affinity DataEC50: 1.40E+6nMAssay Description:Binding affinity to rat bile acid-sensitive ion channel expressed in xenopus oocytesMore data for this Ligand-Target Pair
Affinity DataEC50: 2.80E+6nMAssay Description:Binding affinity to rat bile acid-sensitive ion channel expressed in xenopus oocytesMore data for this Ligand-Target Pair
Affinity DataEC50: 2.60E+6nMAssay Description:Binding affinity to rat bile acid-sensitive ion channel expressed in xenopus oocytesMore data for this Ligand-Target Pair

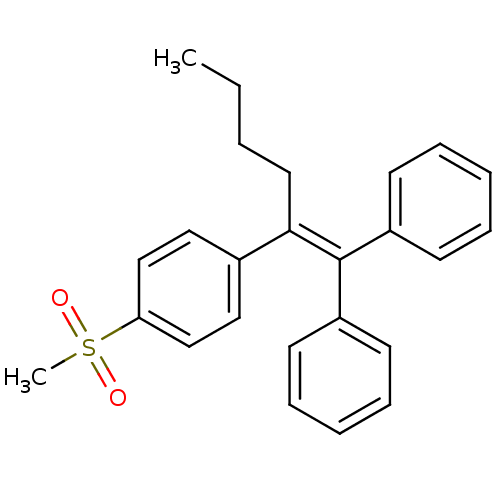
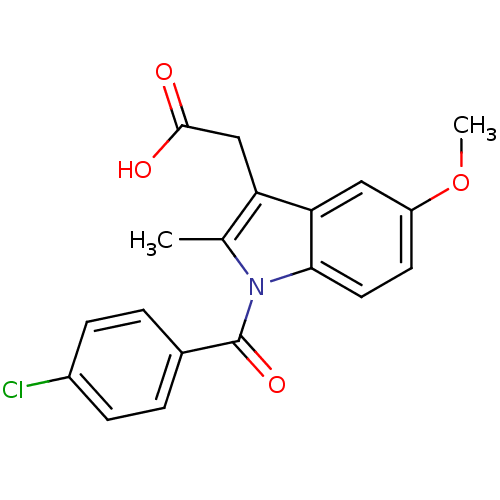
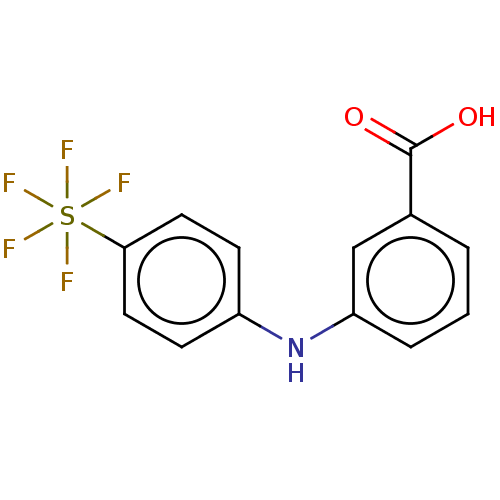
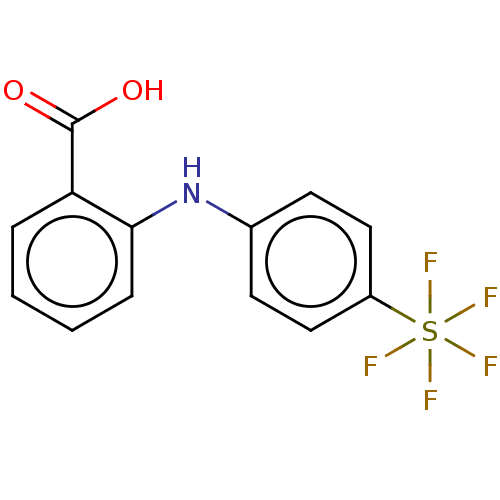
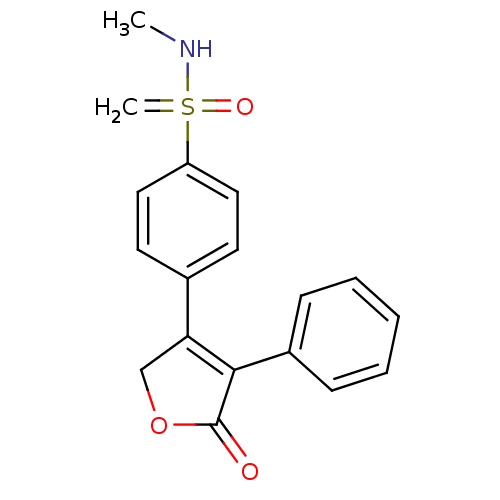

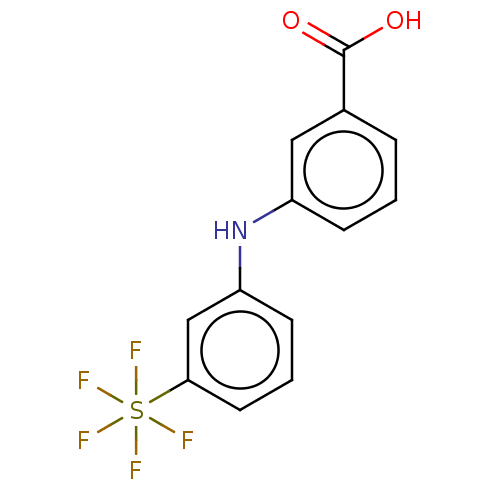
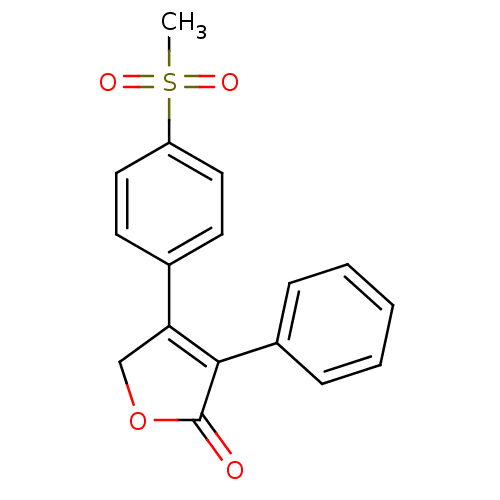
 3D Structure (crystal)
3D Structure (crystal)