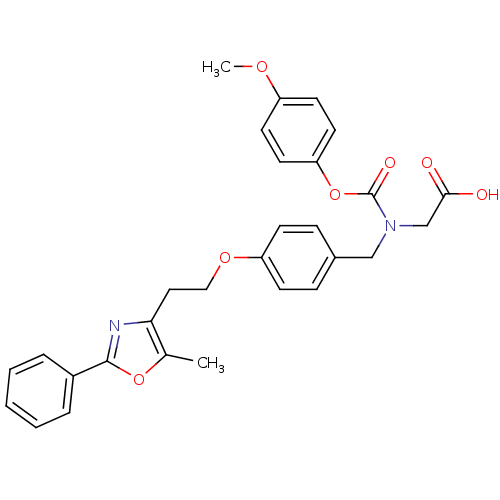
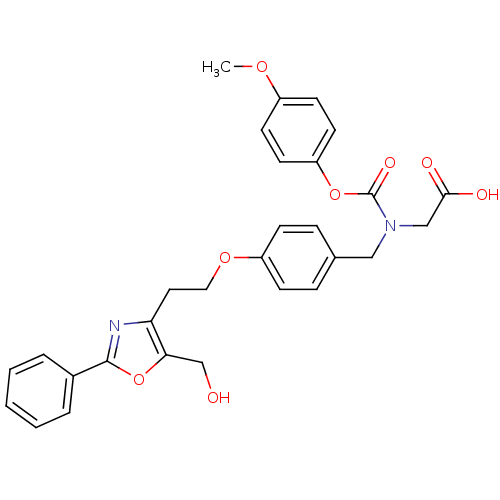
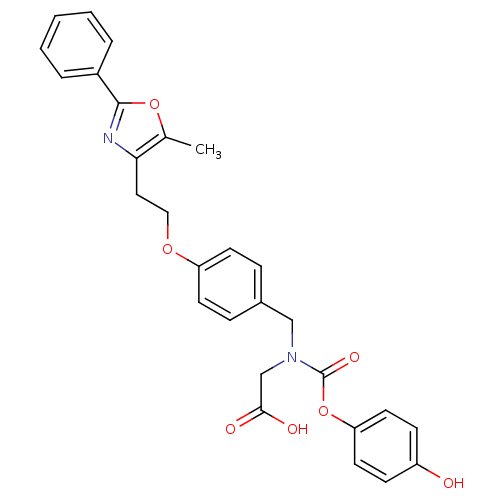
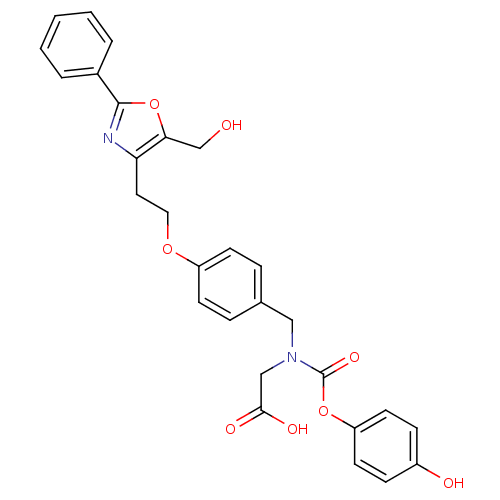
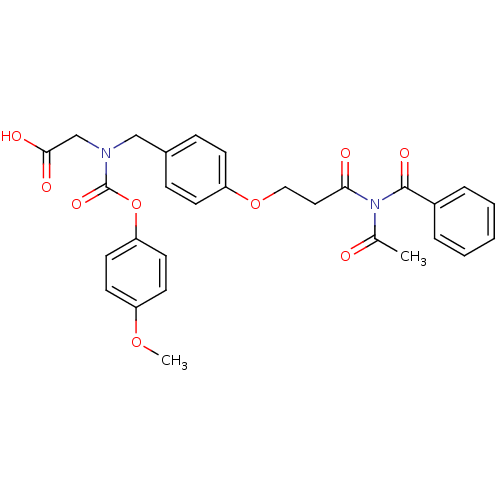
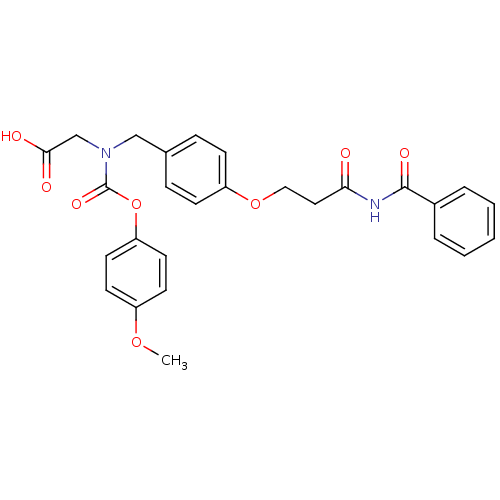
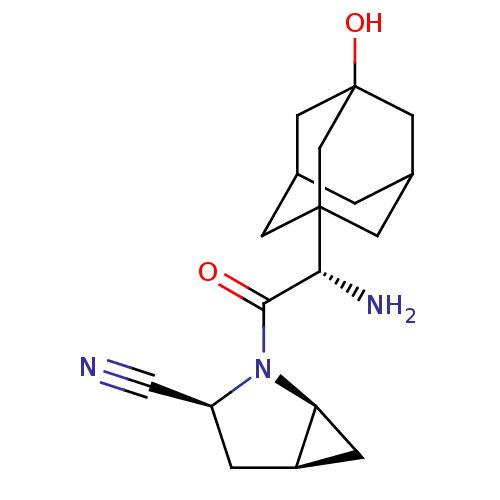
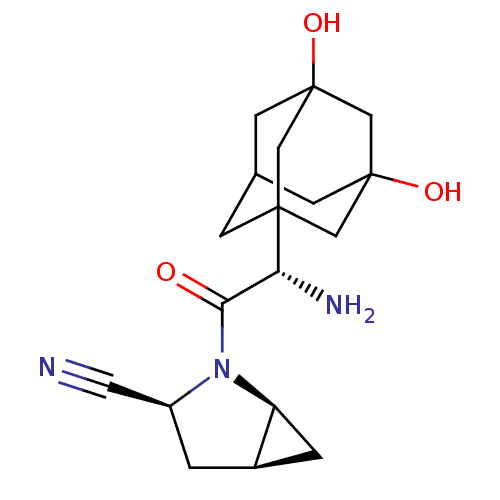
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: 99nMAssay Description:Binding affinity at PPAR alpha receptorMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: 188nMAssay Description:Binding affinity at PPAR alpha receptorMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: 247nMAssay Description:Binding affinity at PPAR gamma receptorMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: 1.41E+3nMAssay Description:Agonist activity at PPAR gamma in human HEK cells by transactivation assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: 2.05E+3nMAssay Description:Binding affinity at PPAR gamma receptorMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: 3.09E+3nMAssay Description:Binding affinity at PPAR alpha receptorMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: 3.74E+3nMAssay Description:Binding affinity at PPAR alpha receptorMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: >1.50E+4nMAssay Description:Binding affinity at PPAR gamma receptorMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: >1.50E+4nMAssay Description:Binding affinity at PPAR alpha receptorMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: >1.50E+4nMAssay Description:Binding affinity at PPAR gamma receptorMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: >1.50E+4nMAssay Description:Binding affinity at PPAR gamma receptorMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: >1.50E+4nMAssay Description:Binding affinity at PPAR gamma receptorMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: >1.50E+4nMAssay Description:Binding affinity at PPAR alpha receptorMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: >1.50E+4nMAssay Description:Binding affinity at PPAR alpha receptorMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: >1.50E+4nMAssay Description:Binding affinity at PPAR gamma receptorMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: >1.50E+4nMAssay Description:Binding affinity at PPAR alpha receptorMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: >1.50E+4nMAssay Description:Binding affinity at PPAR gamma receptorMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: 1.98E+4nMAssay Description:Agonist activity at PPAR gamma in human HEK cells by transactivation assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Agonist activity at PPAR gamma in human HEK cells by transactivation assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: >2.50E+4nMAssay Description:Agonist activity at PPAR gamma in human HEK cells by transactivation assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: >2.50E+4nMAssay Description:Agonist activity at PPAR gamma in human HEK cells by transactivation assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: >2.50E+4nMAssay Description:Agonist activity at PPAR gamma in human HEK cells by transactivation assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: >2.50E+4nMAssay Description:Agonist activity at PPAR gamma in human HEK cells by transactivation assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: >2.50E+4nMAssay Description:Agonist activity at PPAR gamma in human HEK cells by transactivation assayMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP1A2 in human liver microsomes assessed as phenacetin O-deethylation after 3 mins by LC-MS/MS analysis in presence of NADPHMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2A6 in human liver microsomes assessed as coumarin 7-hydroxylation after 3 mins by LC-MS/MS analysis in presence of NADPHMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2B6 in human liver microsomes assessed as bupropion hydroxylation after 3 mins by LC-MS/MS analysis in presence of NADPHMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2C8 in human liver microsomes assessed as paclitaxel 6alpha-hydroxylation after 3 mins by LC-MS/MS analysis in presence of NADPHMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2C9 in human liver microsomes assessed as paclitaxel 6alpha-hydroxylation after 3 mins by LC-MS/MS analysis in presence of NADPHMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2C19 in human liver microsomes assessed as (S)-mephenytoin 4'-hydroxylation after 3 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2D6 in human liver microsomes assessed as bufuralol 1'-hydroxylation after 3 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2E1 in human liver microsomes assessed as chlorzoxazone 6-hydroxylation after 3 mins by LC-MS/MS analysis in presence of NADPHMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP3A4 in human liver microsomes assessed as midazolam 1'-hydroxylation after 3 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP3A4 in human liver microsomes assessed as testosterone 6beta-hydroxylation after 3 mins by LC-MS/MS analysis in presence of NADPHMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP1A2 in human liver microsomes assessed as phenacetin O-deethylation preincubated for 15 mins by LC-MS/MS analysis in presence of NAD...More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2A6 in human liver microsomes assessed as coumarin 7-hydroxylation preincubated for 15 mins by LC-MS/MS analysis in presence of NADP...More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2B6 in human liver microsomes assessed as bupropion hydroxylation preincubated for 15 mins by LC-MS/MS analysis in presence of NADPHMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2C8 in human liver microsomes assessed as paclitaxel 6alpha-hydroxylation preincubated for 15 mins by LC-MS/MS analysis in presence ...More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2C9 in human liver microsomes assessed as paclitaxel 6alpha-hydroxylation preincubated for 15 mins by LC-MS/MS analysis in presence ...More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2C19 in human liver microsomes assessed as (S)-mephenytoin 4'-hydroxylation preincubated for 15 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2D6 in human liver microsomes assessed as bufuralol 1'-hydroxylation preincubated for 15 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2E1 in human liver microsomes assessed as chlorzoxazone 6-hydroxylation preincubated for 15 mins by LC-MS/MS analysis in presence of...More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP3A4 in human liver microsomes assessed as midazolam 1'-hydroxylation preincubated for 15 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP3A4 in human liver microsomes assessed as testosterone 6beta-hydroxylation preincubated for 15 mins by LC-MS/MS analysis in presence...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibition of CYP1A2 in human liver microsomes assessed as phenacetin O-deethylation after 3 mins by LC-MS/MS analysis in presence of NADPHMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibition of CYP2A6 in human liver microsomes assessed as coumarin 7-hydroxylation after 3 mins by LC-MS/MS analysis in presence of NADPHMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibition of CYP3A4 in human liver microsomes assessed as testosterone 6beta-hydroxylation preincubated for 15 mins by LC-MS/MS analysis in presence...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibition of CYP3A4 in human liver microsomes assessed as midazolam 1'-hydroxylation preincubated for 15 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibition of CYP2E1 in human liver microsomes assessed as chlorzoxazone 6-hydroxylation preincubated for 15 mins by LC-MS/MS analysis in presence of...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibition of CYP2D6 in human liver microsomes assessed as bufuralol 1'-hydroxylation preincubated for 15 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair