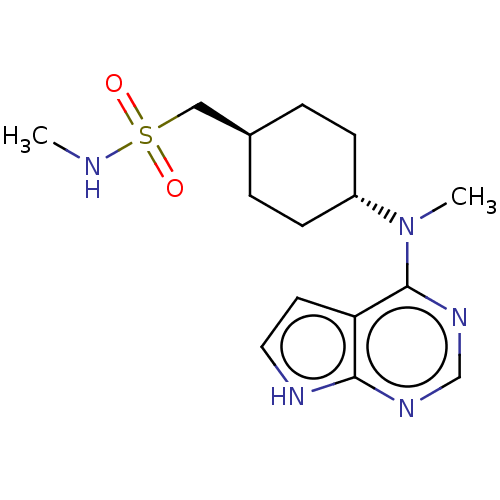
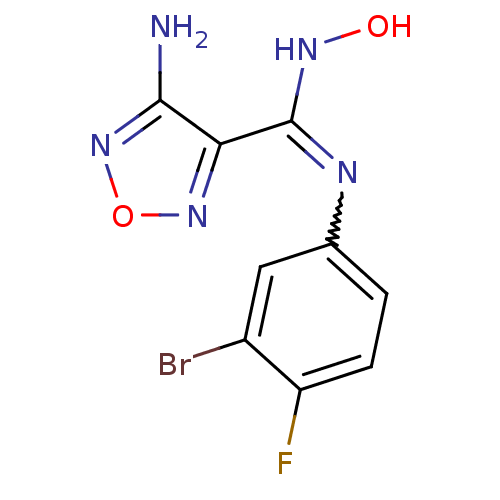
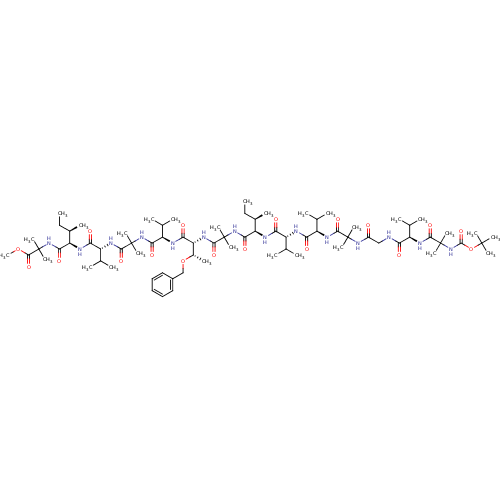
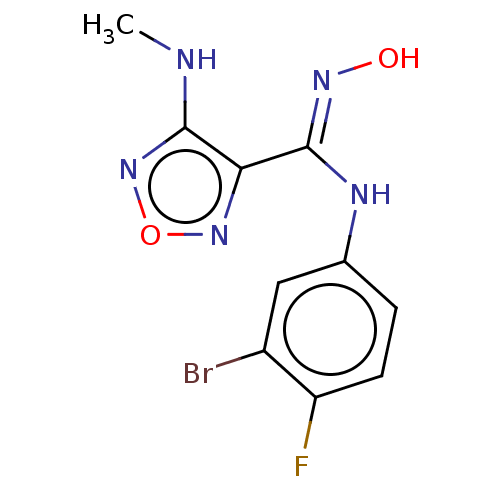
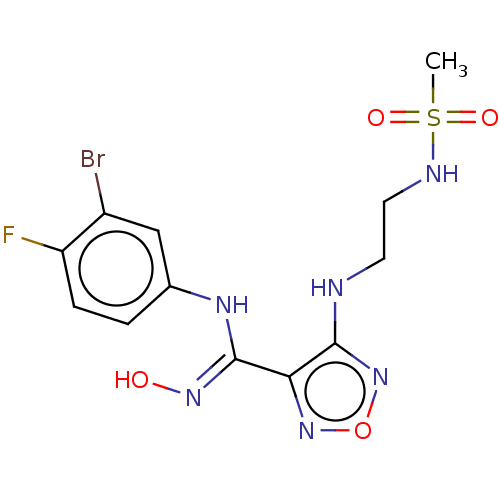
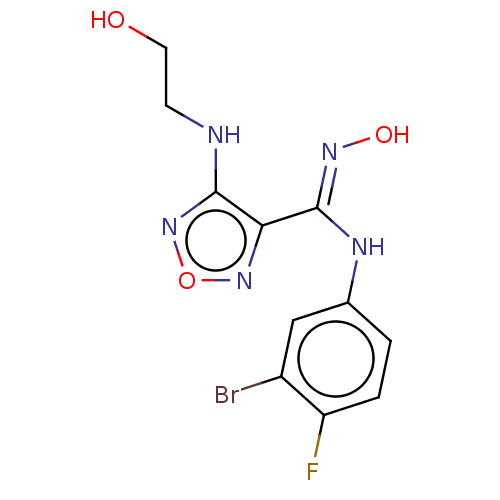
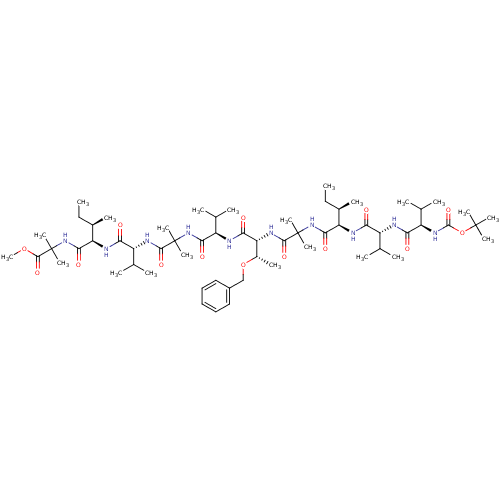
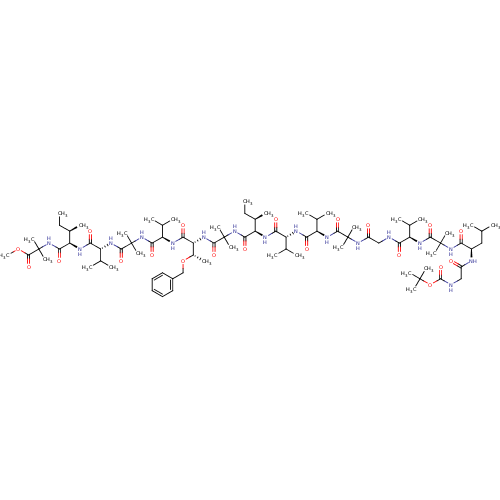
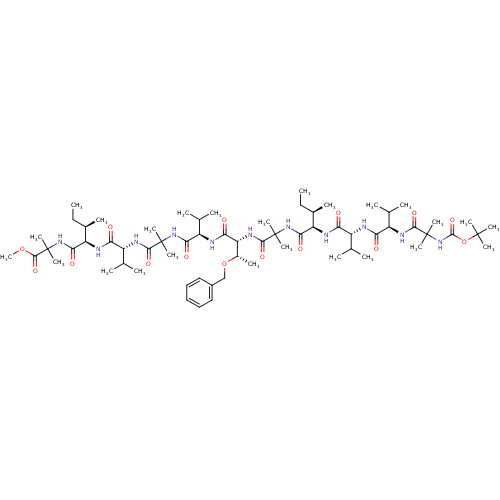
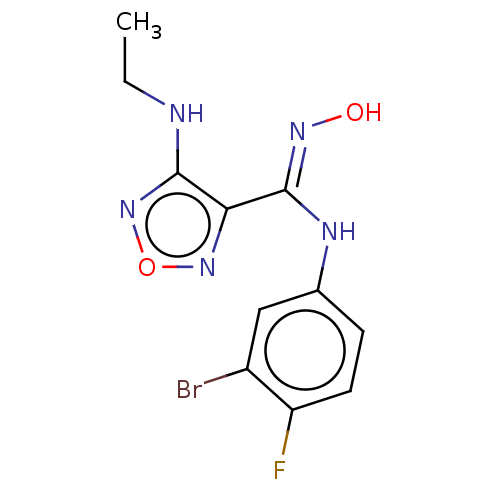
TargetCapsid scaffolding protein(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...)
Purdue University
Purdue University
Affinity DataKi: 96nM IC50: 360nMpH: 5.52Assay Description:A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor.More data for this Ligand-Target Pair
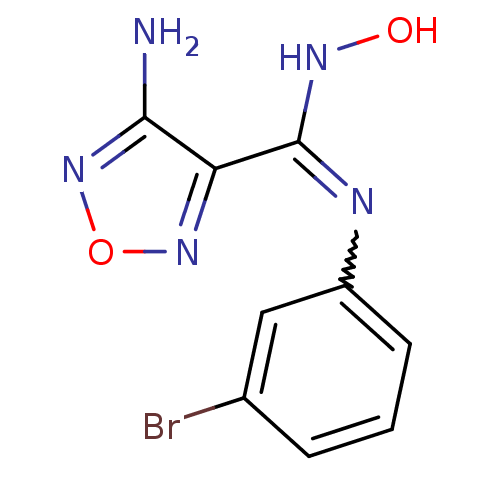
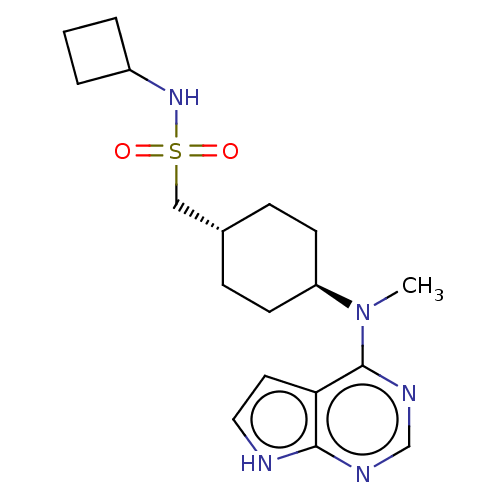
TargetCapsid scaffolding protein(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...)
Purdue University
Purdue University
Affinity DataKi: 175nM IC50: 730nMpH: 5.52Assay Description:A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor.More data for this Ligand-Target Pair
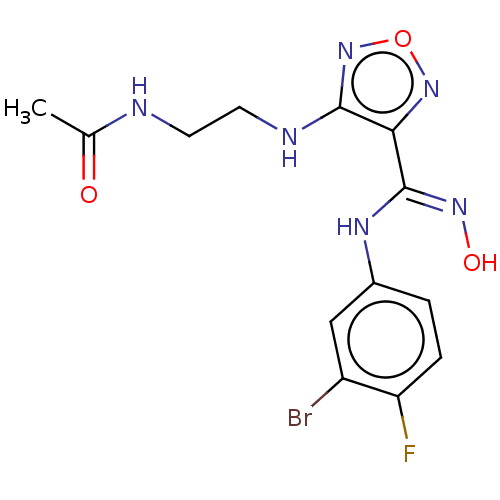
TargetCapsid scaffolding protein(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...)
Purdue University
Purdue University
Affinity DataKi: 220nM IC50: 350nMpH: 5.52Assay Description:The competitive assay requires two inhibitors to act by a purely competitive mechanism, whereas the binding site of on the inhibitors has been establ...More data for this Ligand-Target Pair
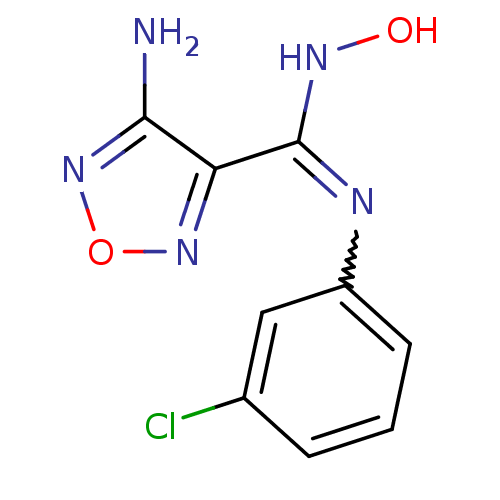
TargetCapsid scaffolding protein(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...)
Purdue University
Purdue University
Affinity DataKi: 600nM IC50: 2.90E+3nMpH: 5.52Assay Description:A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor.More data for this Ligand-Target Pair
TargetCapsid scaffolding protein(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...)
Purdue University
Purdue University
Affinity DataKi: 650nM IC50: 2.50E+3nMpH: 5.52Assay Description:A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor.More data for this Ligand-Target Pair
TargetCapsid scaffolding protein(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...)
Purdue University
Purdue University
Affinity DataKi: 720nM IC50: 2.70E+3nMpH: 5.52Assay Description:A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor.More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec...More data for this Ligand-Target Pair
TargetCapsid scaffolding protein(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...)
Purdue University
Purdue University
Affinity DataKi: 1.13E+3nM IC50: 4.40E+3nMpH: 5.52Assay Description:The competitive assay requires two inhibitors to act by a purely competitive mechanism, whereas the binding site of on the inhibitors has been establ...More data for this Ligand-Target Pair
Affinity DataKi: 1.25E+3nMAssay Description:Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assayMore data for this Ligand-Target Pair
TargetCapsid scaffolding protein(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...)
Purdue University
Purdue University
Affinity DataKi: 1.29E+3nM IC50: 4.90E+3nMpH: 5.52Assay Description:The competitive assay requires two inhibitors to act by a purely competitive mechanism, whereas the binding site of on the inhibitors has been establ...More data for this Ligand-Target Pair
Affinity DataKi: 1.30E+3nMAssay Description:Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.30E+3nMAssay Description:Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assayMore data for this Ligand-Target Pair
TargetCapsid scaffolding protein(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...)
Purdue University
Purdue University
Affinity DataKi: 3.00E+3nM IC50: 5.90E+3nMpH: 5.52Assay Description:The competitive assay requires two inhibitors to act by a purely competitive mechanism, whereas the binding site of on the inhibitors has been establ...More data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assayMore data for this Ligand-Target Pair
Affinity DataKi: 6.80E+3nMAssay Description:Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assayMore data for this Ligand-Target Pair
Affinity DataKi: 8.30E+3nMAssay Description:Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.69E+4nMAssay Description:Inhibition of HIV1 protease by competitive inhibition assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.40E+4nMAssay Description:Competitive inhibition of IDO1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 4.01E+4nMAssay Description:Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assayMore data for this Ligand-Target Pair
Affinity DataKi: 8.80E+4nMAssay Description:Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assayMore data for this Ligand-Target Pair
Affinity DataKi: 5.10E+5nMAssay Description:Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assayMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataIC50: 0.140nMAssay Description:Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporterMore data for this Ligand-Target Pair
Affinity DataIC50: 7.40nMAssay Description:Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrsMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibitory activity against Gamma-secretase from APP-transfected CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 9.53nMpH: 7.4Assay Description:Methods: A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK-2 and JAK-3) or the IRS-1 peptide (JAK-1 and Tyk...More data for this Ligand-Target Pair
Affinity DataIC50: 9.53nMpH: 7.4Assay Description:Methods: A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK-2 and JAK-3) or the IRS-1 peptide (JAK-1 and Tyk...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrsMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporterMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometryMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporterMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrsMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporterMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 17.5nMpH: 7.4Assay Description:Methods: A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK-2 and JAK-3) or the IRS-1 peptide (JAK-1 and Tyk...More data for this Ligand-Target Pair
Affinity DataIC50: 17.5nMpH: 7.4Assay Description:Methods: A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK-2 and JAK-3) or the IRS-1 peptide (JAK-1 and Tyk...More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrsMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporterMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporterMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataIC50: 28nMAssay Description:Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporterMore data for this Ligand-Target Pair
Affinity DataIC50: 35nMAssay Description:Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMpH: 7.4Assay Description:Methods: A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK-2 and JAK-3) or the IRS-1 peptide (JAK-1 and Tyk...More data for this Ligand-Target Pair
Affinity DataIC50: 45nMpH: 7.4Assay Description:Methods: A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK-2 and JAK-3) or the IRS-1 peptide (JAK-1 and Tyk...More data for this Ligand-Target Pair
Affinity DataIC50: 46nMAssay Description:Inhibition of indoleamine 2,3-dioxygenase in mouse B16 cells assessed as kynurenine formation by spectrophotometryMore data for this Ligand-Target Pair


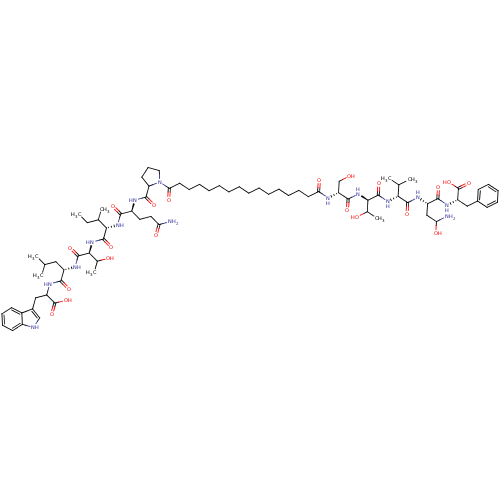

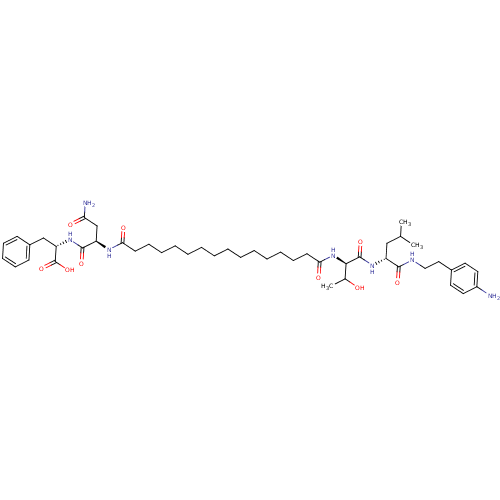
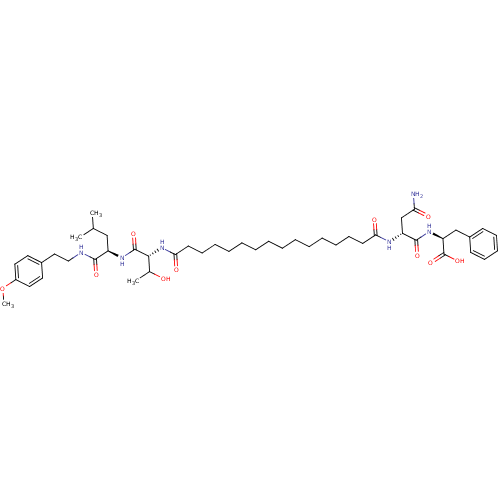
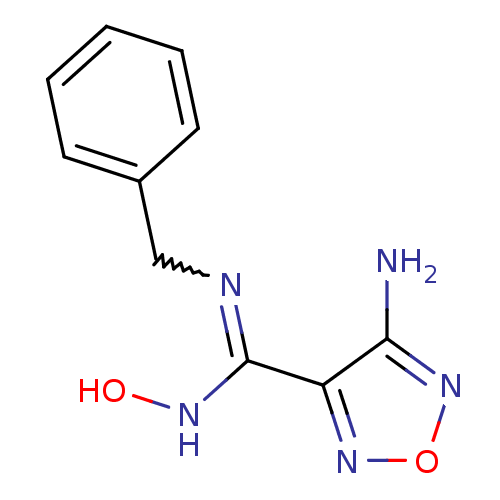
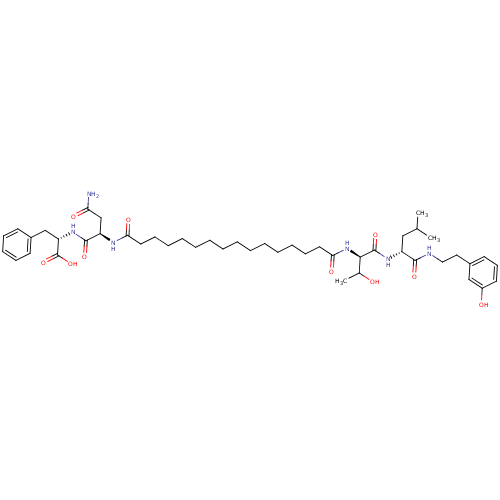
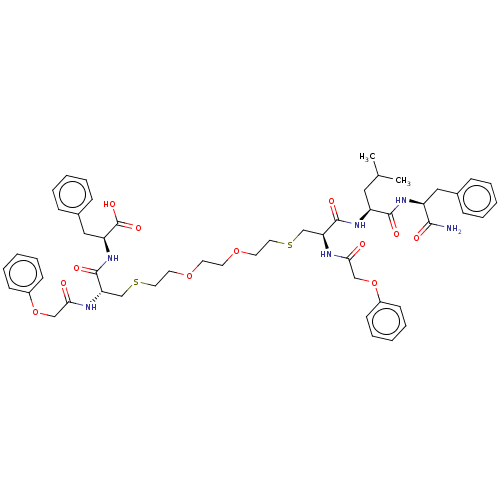
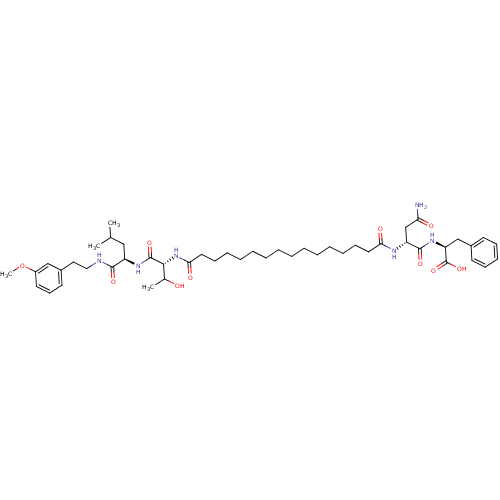
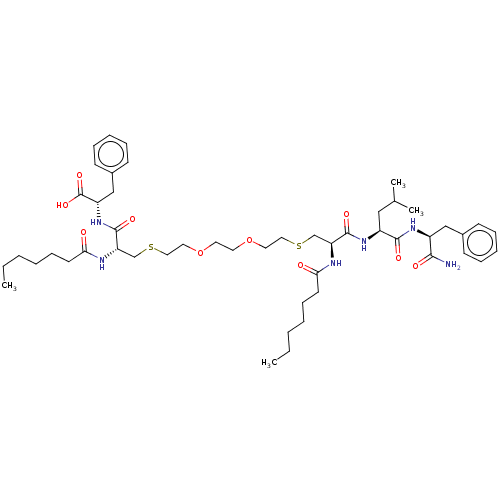
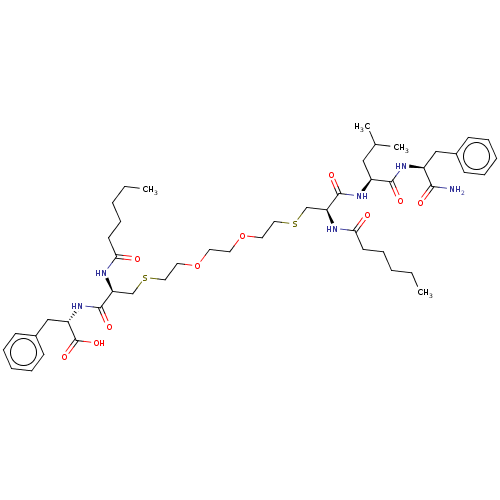
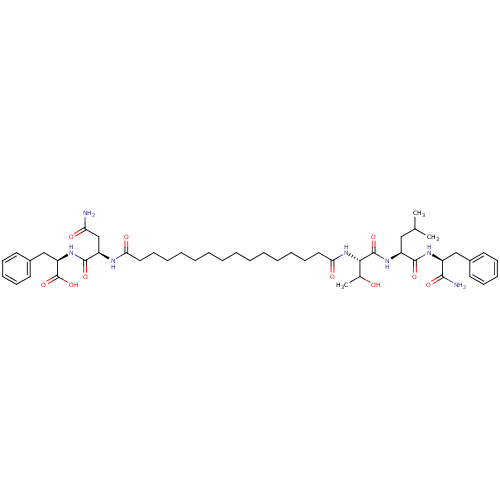

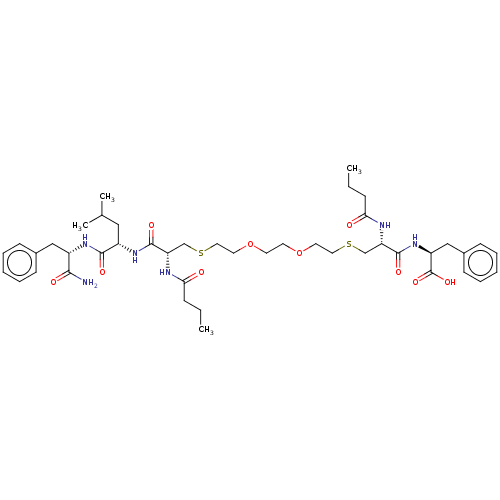
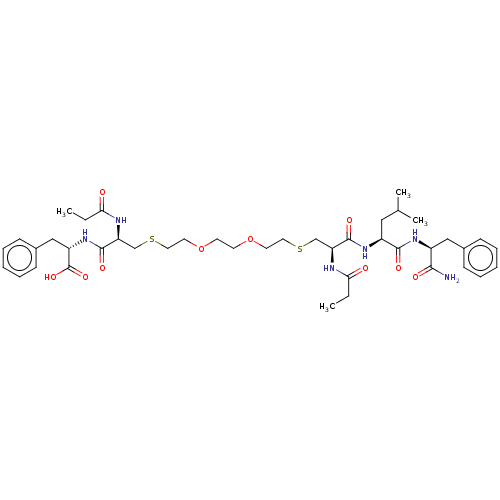
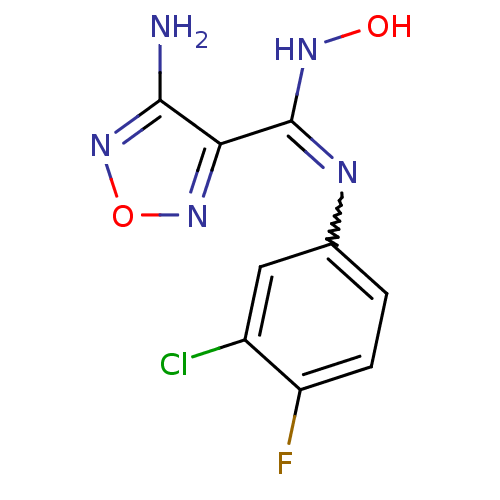
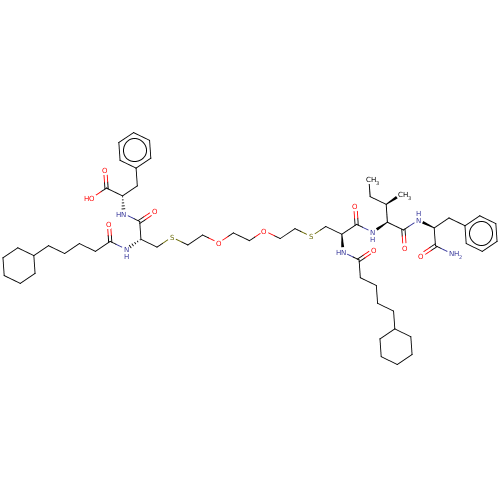


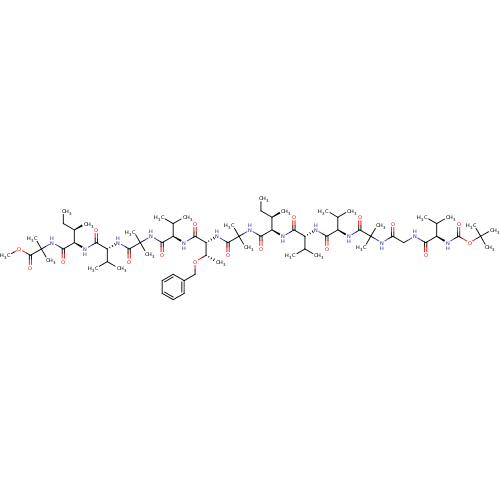
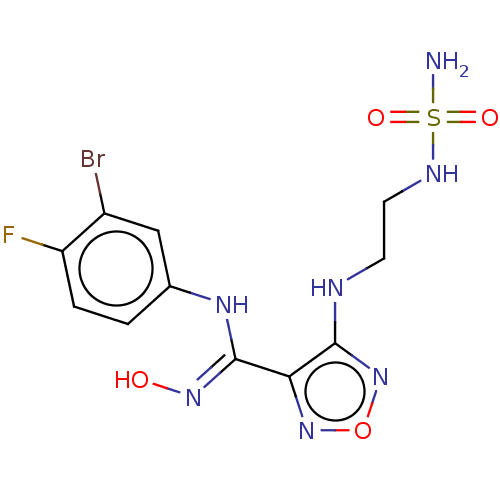
 3D Structure (crystal)
3D Structure (crystal)