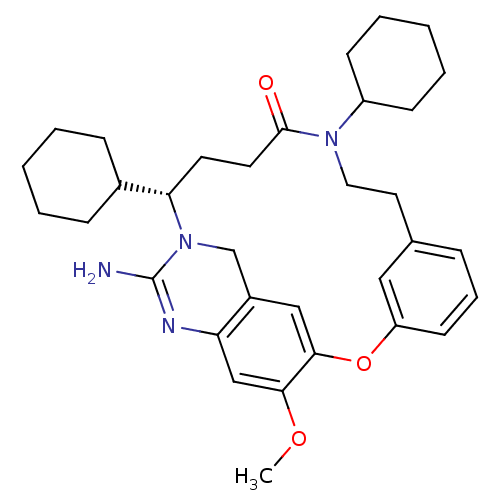
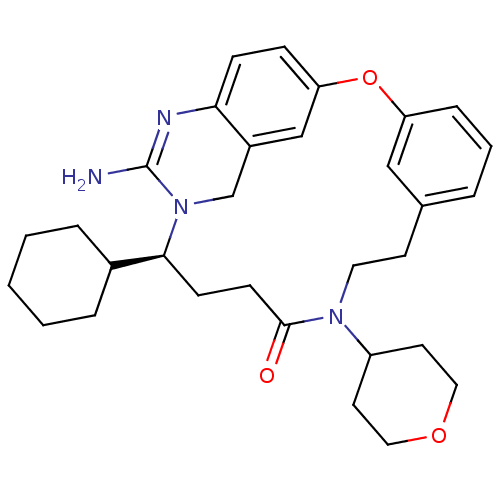
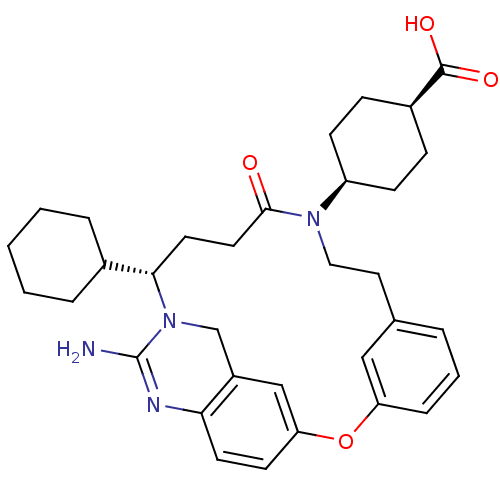
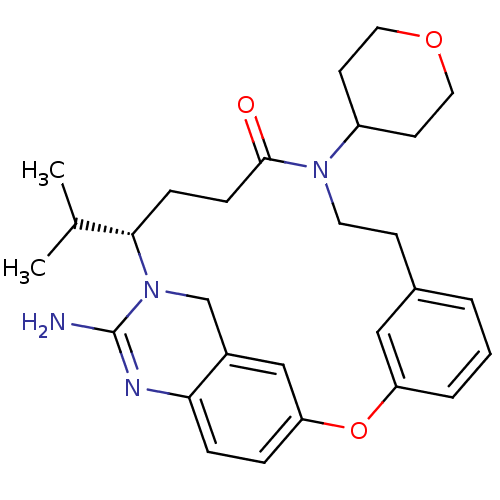
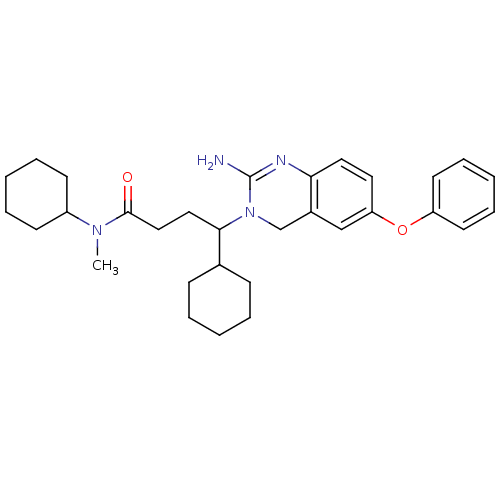
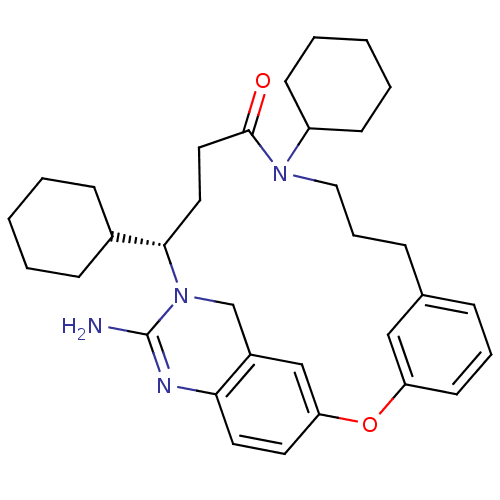
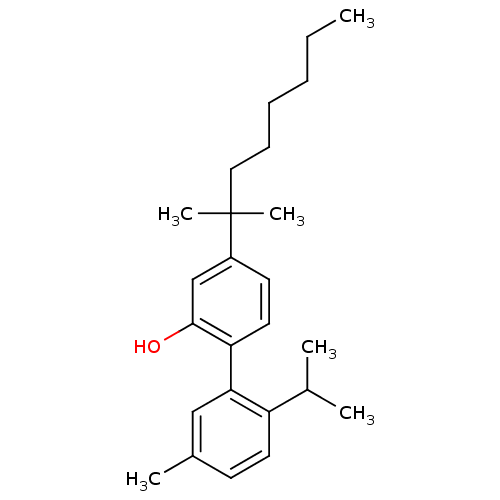
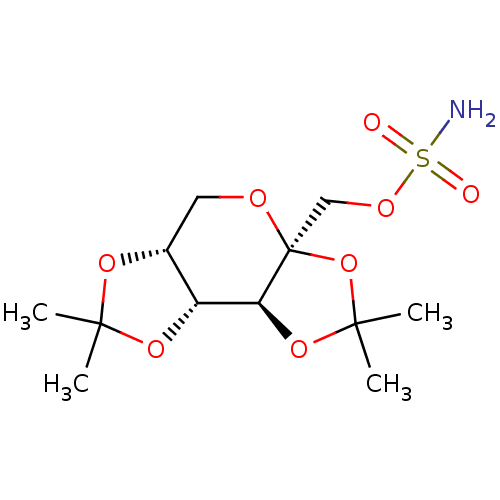
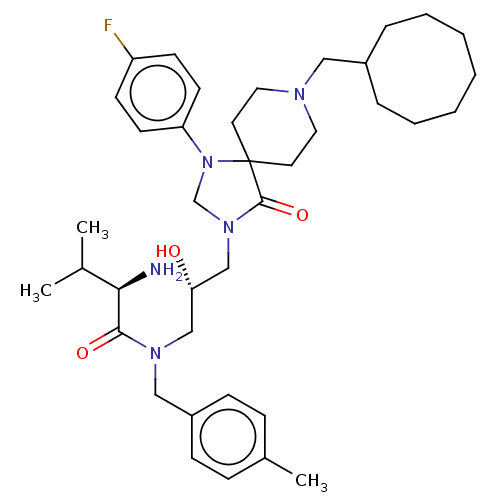
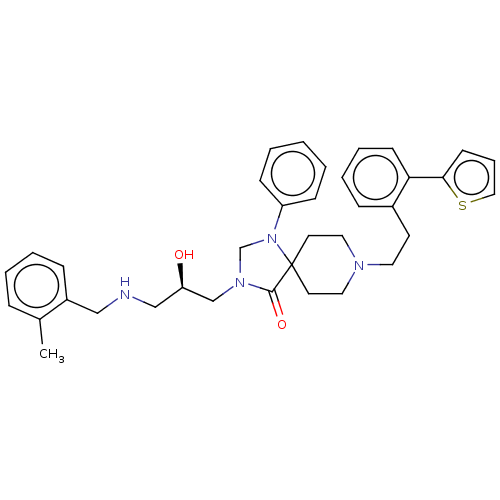
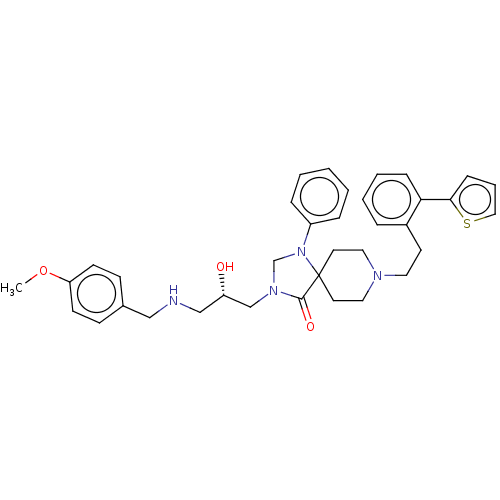
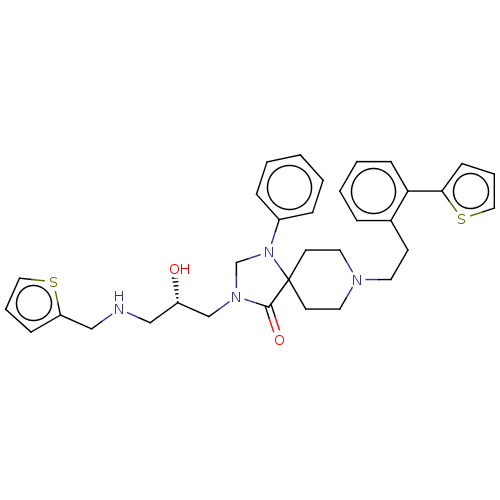
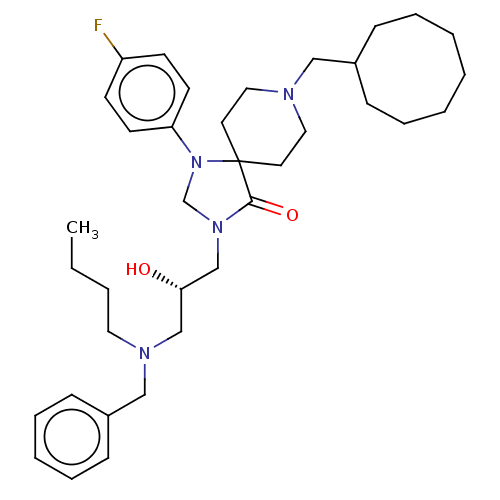
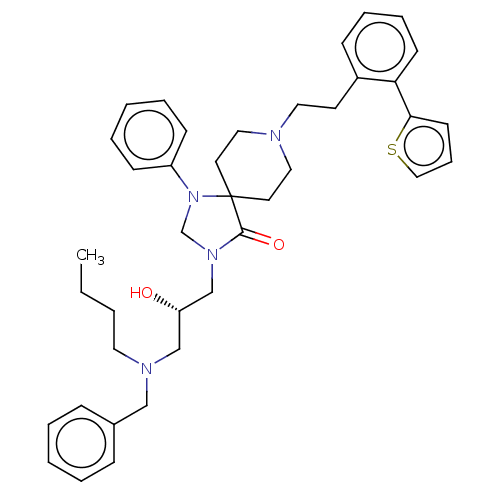
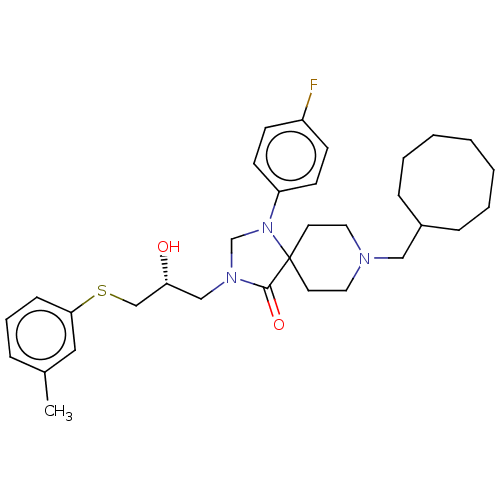
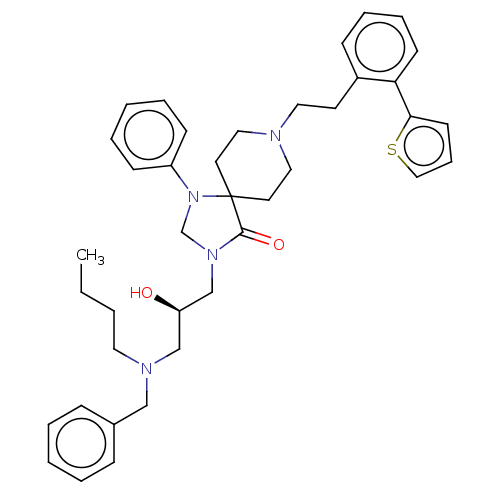
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
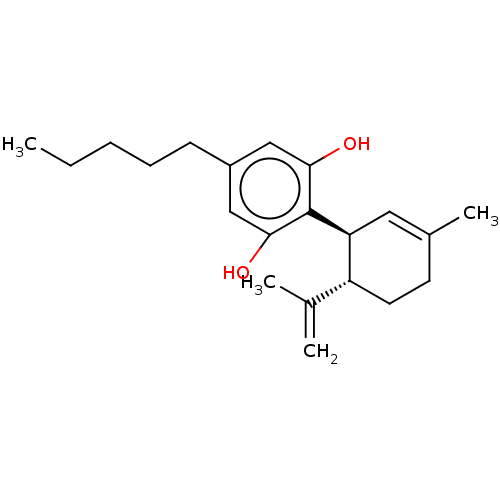
Affinity DataKi: 11nMAssay Description:Displacement of [3H]CP 55940 from human CB1 receptor after 1 hr by liquid scintillation spectrometryMore data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
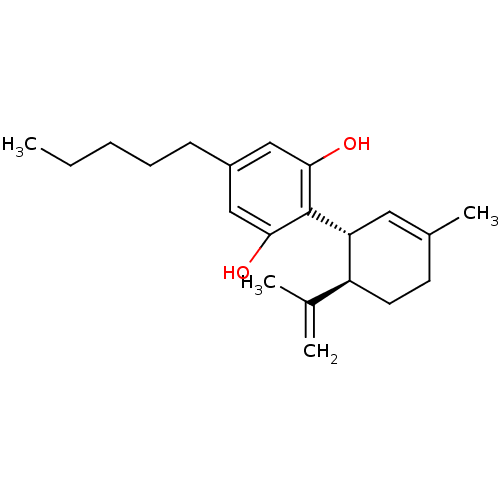
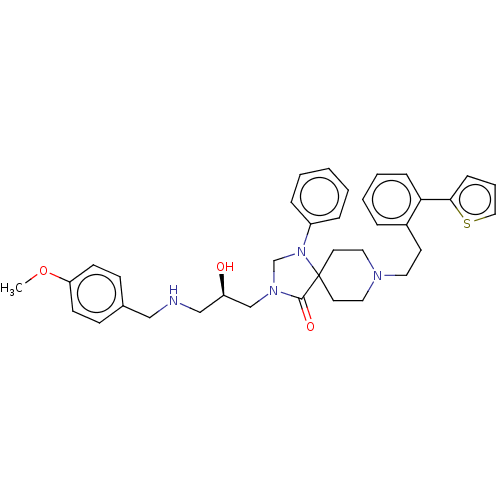
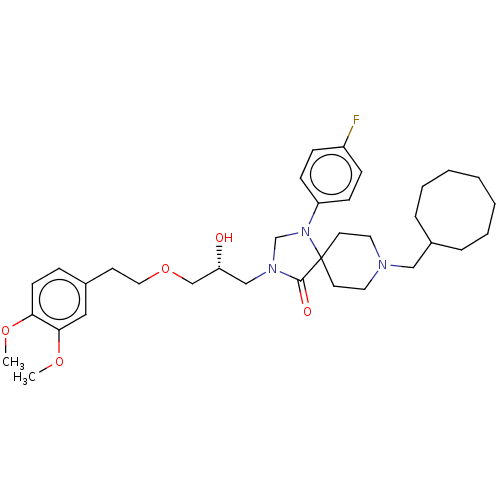
Affinity DataKi: 11nM ΔG°: -45.0kJ/molepH: 5.0 T: 2°CAssay Description:BACE-1 activity was measured at pH 5 using the FS1 FRET substrate. Compounds were preincubated with recombinant BACE-1 for 20 min before adding subst...More data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
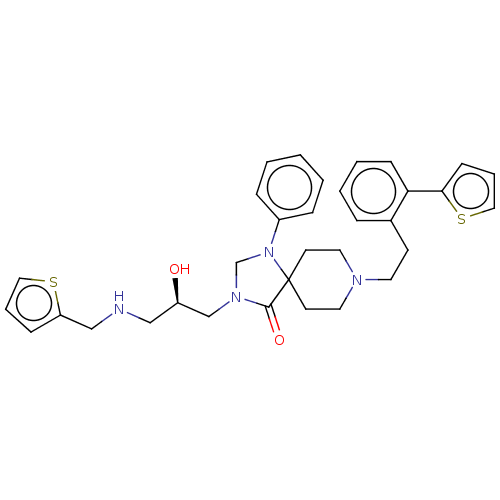
Affinity DataKi: 30nM ΔG°: -42.5kJ/molepH: 5.0 T: 2°CAssay Description:BACE-1 activity was measured at pH 5 using the FS1 FRET substrate. Compounds were preincubated with recombinant BACE-1 for 20 min before adding subst...More data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 33nMAssay Description:Displacement of [3H]CP 55940 from human CB1 receptor after 1 hr by liquid scintillation spectrometryMore data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
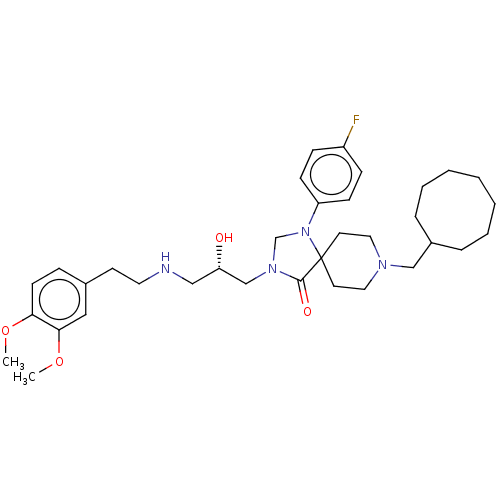
Affinity DataKi: 158nM ΔG°: -38.4kJ/molepH: 5.0 T: 2°CAssay Description:BACE-1 activity was measured at pH 5 using the FS1 FRET substrate. Compounds were preincubated with recombinant BACE-1 for 20 min before adding subst...More data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
TargetCarbonic anhydrase 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
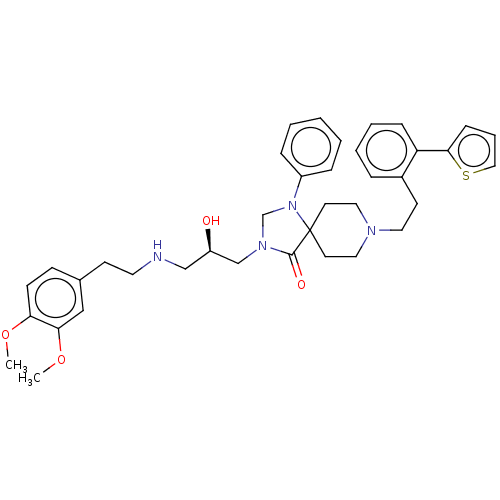
Affinity DataKi: 300nMAssay Description:Inhibition of human carbonic anhydrase 2 by ThermoFluor methodMore data for this Ligand-Target Pair
Affinity DataKi: 842nMAssay Description:Displacement of [3H]-HU-243 from CB1 receptor in Sabra rat brain synaptosomes after 90 minsMore data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
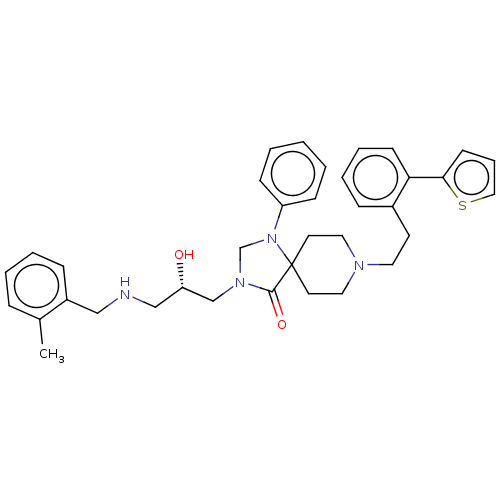
Affinity DataKi: 900nM ΔG°: -34.2kJ/molepH: 5.0 T: 2°CAssay Description:BACE-1 activity was measured at pH 5 using the FS1 FRET substrate. Compounds were preincubated with recombinant BACE-1 for 20 min before adding subst...More data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of BACE1More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]-HU-243 from CB1 receptor in Sabra rat brain synaptosomes after 90 minsMore data for this Ligand-Target Pair
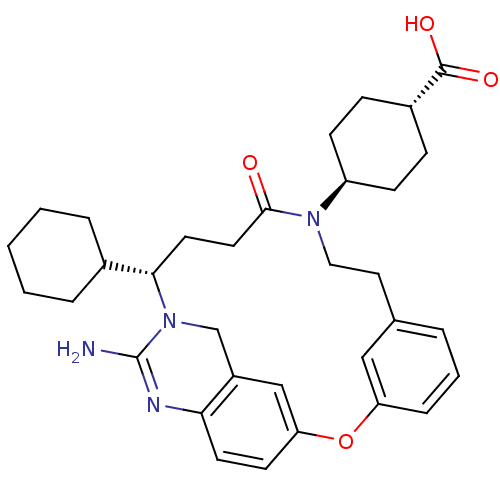
Affinity DataIC50: 3.90nMAssay Description:Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 4.20nMAssay Description:Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of BACE1 assessed as amyloid beta (1-40) productionMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 8.80nMAssay Description:Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of BACE1 assessed as amyloid beta (1-40) productionMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Displacement of [3H]DAMGO from human Mu opioid receptor expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Displacement of [3H]DAMGO from human Mu opioid receptor expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of BACE1 assessed as amyloid beta (1-40) productionMore data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Inhibition of BACE1 assessed as amyloid beta (1-40) productionMore data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Displacement of [3H]DAMGO from human Mu opioid receptor expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 36nMAssay Description:Inhibition of BACE1 assessed as amyloid beta (1-40) productionMore data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 47nMAssay Description:Displacement of [3H]DAMGO from human Mu opioid receptor expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 57nMAssay Description:Displacement of [3H]DAMGO from human Mu opioid receptor expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 61nMAssay Description:Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 69nMAssay Description:Displacement of [3H]DAMGO from human Mu opioid receptor expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 73nMAssay Description:Inhibition of BACE1 assessed as amyloid beta (1-40) productionMore data for this Ligand-Target Pair
Affinity DataIC50: 79nMAssay Description:Displacement of [3H]DAMGO from human Mu opioid receptor expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Displacement of [3H]DAMGO from human Mu opioid receptor expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 82nMAssay Description:Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 90nMAssay Description:Inhibition of BACE1 assessed as amyloid beta (1-40) productionMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 103nMAssay Description:Displacement of [3H]DAMGO from human Mu opioid receptor expressed in HEK293 cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 110nMpH: 4.0 T: 2°CAssay Description:Cathepsin D activity was measured at pH 4 using a FRET peptide substrate. Compounds were preincubated with recombinant human liver cathepsin D for 20...More data for this Ligand-Target Pair






 3D Structure (crystal)
3D Structure (crystal)