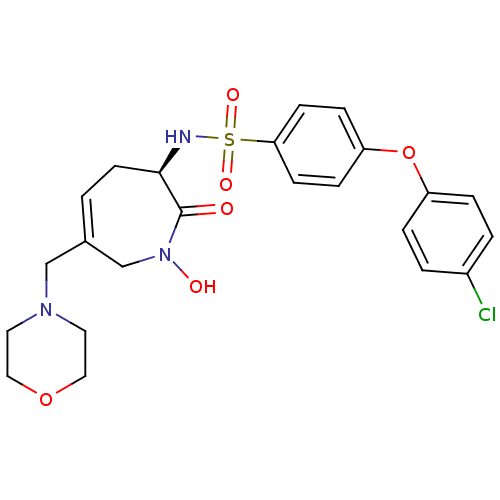
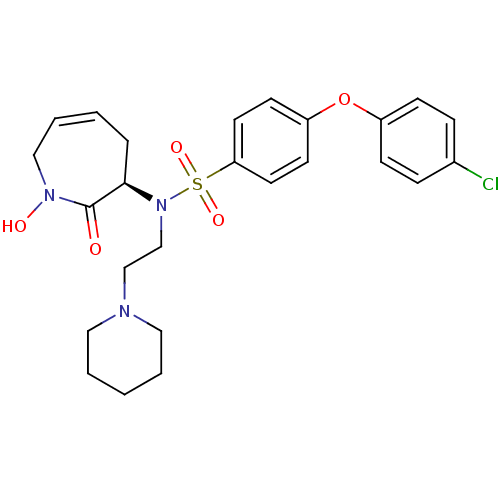
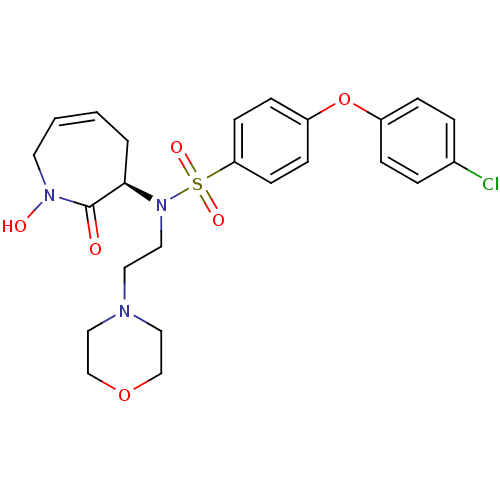
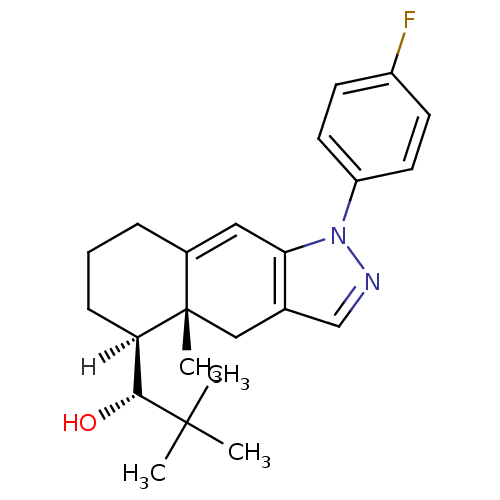
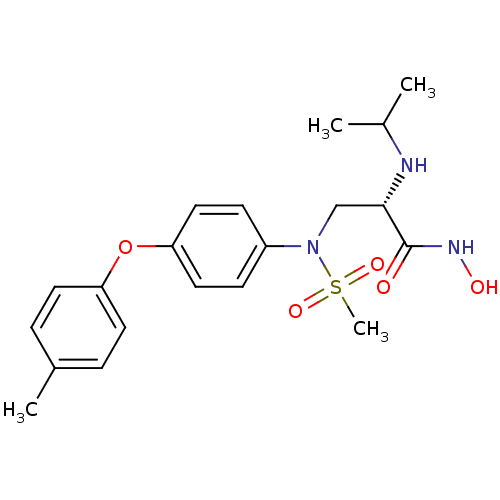
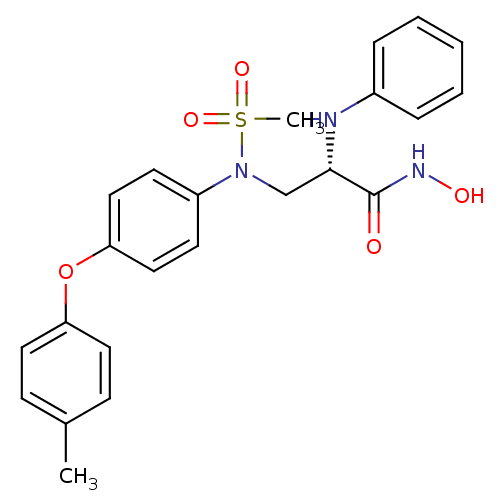
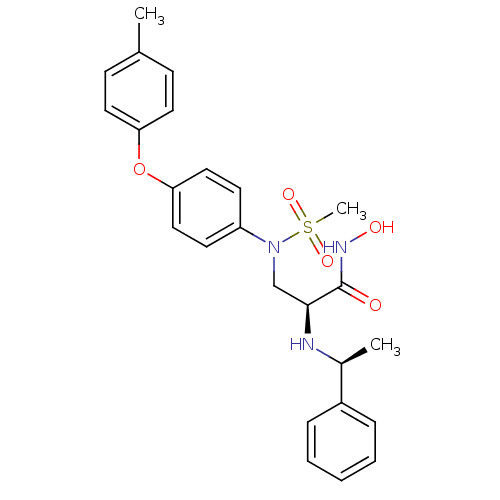
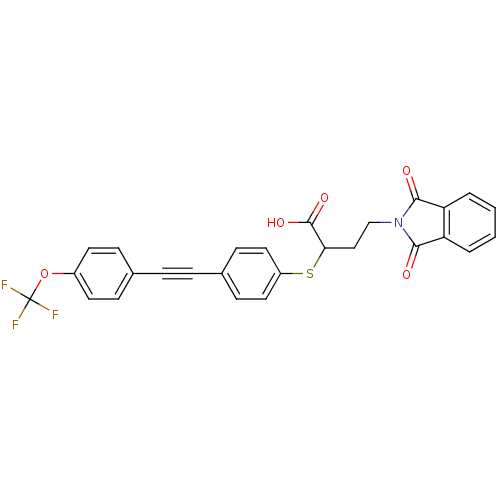
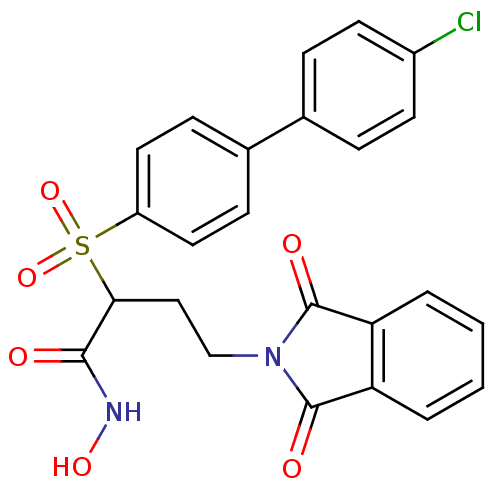
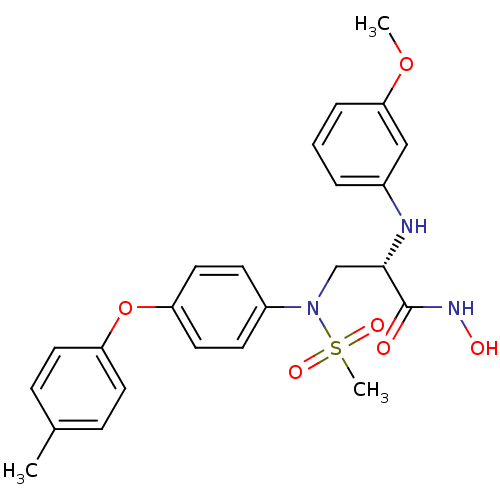
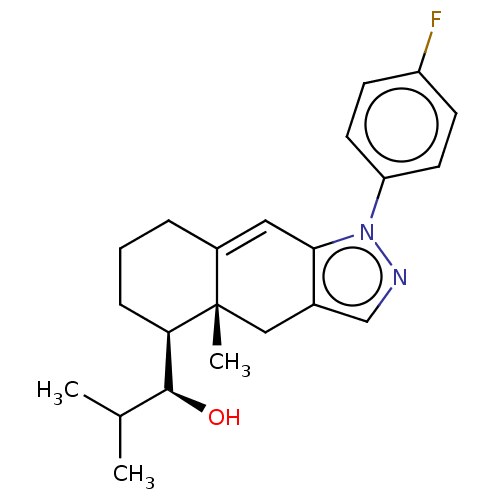
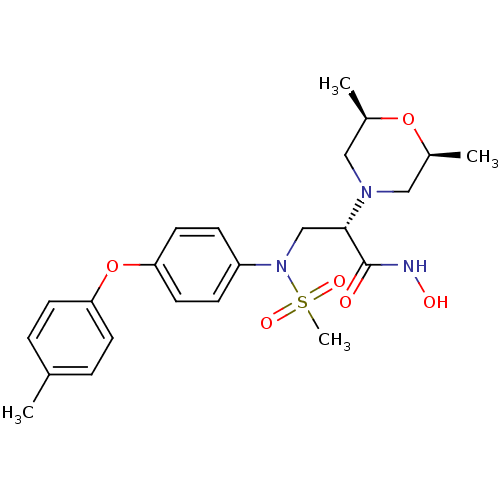
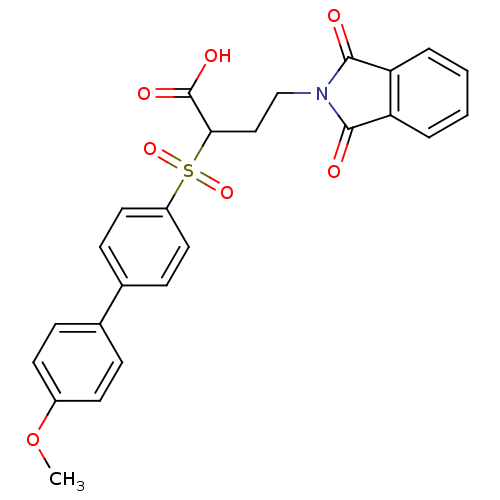
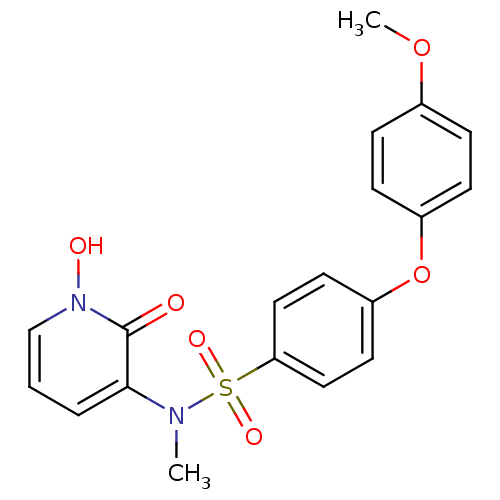
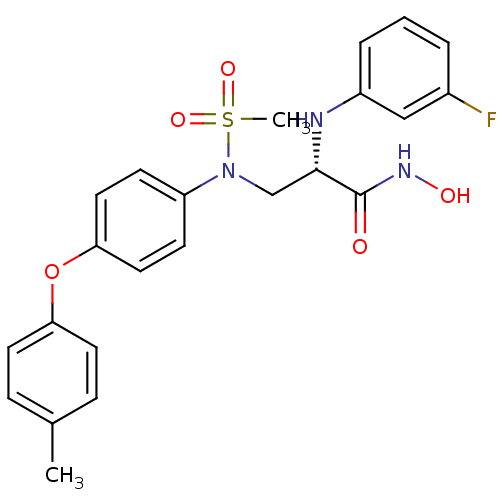
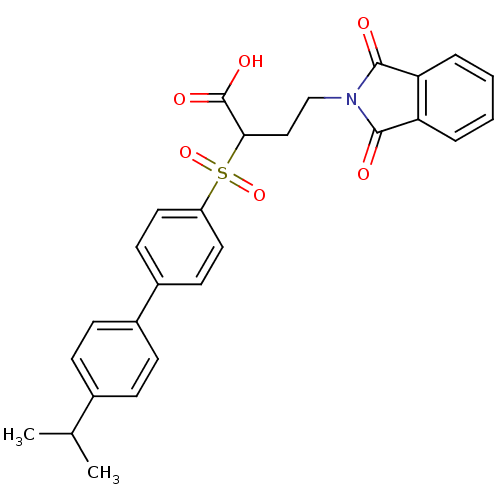
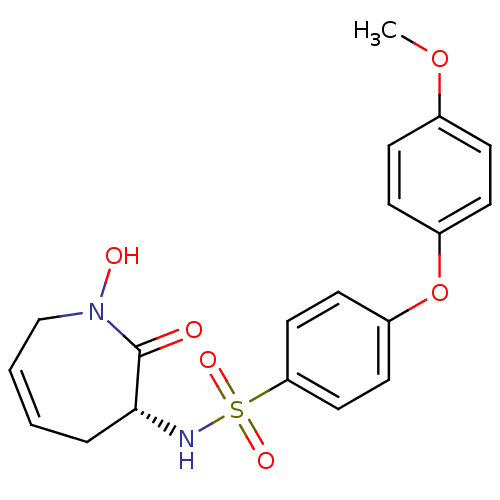
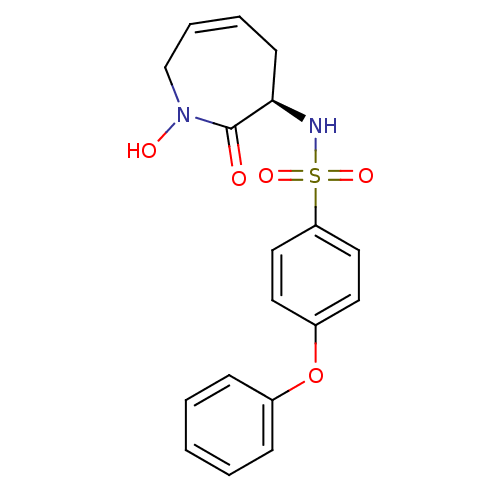
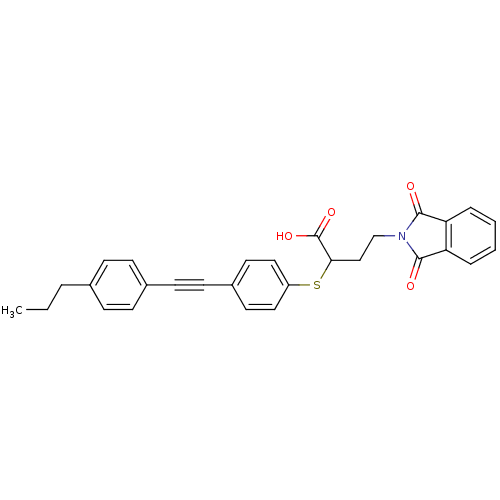
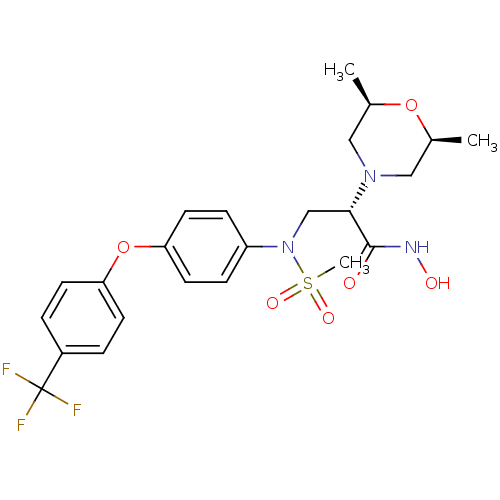
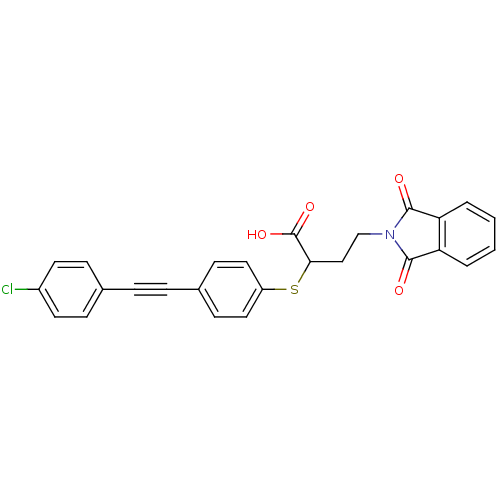
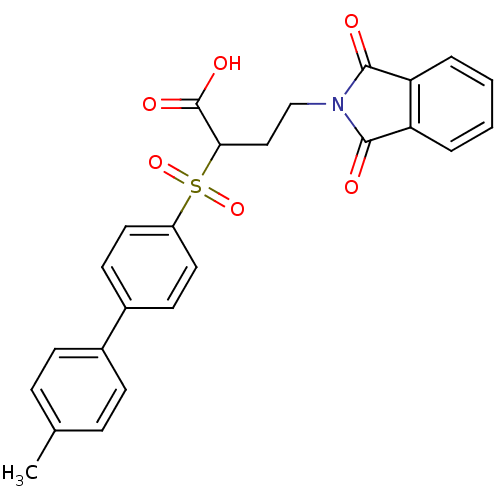
Target72 kDa type IV collagenase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Target72 kDa type IV collagenase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetCollagenase 3(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetCollagenase 3(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.158nMAssay Description:Agonist activity at glucocorticoid receptor in rat INS-1 832/13 cells transfected with CCL2-promoter luciferase plasmid construct assessed as reducti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Agonist activity at glucocorticoid receptor in rat INS-1 832/13 cells transfected with CCL2-promoter luciferase plasmid construct assessed as reducti...More data for this Ligand-Target Pair
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu...More data for this Ligand-Target Pair
TargetCollagenase 3(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human MMP13 catalytic domain (amino acids 103 to 268) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preinc...More data for this Ligand-Target Pair
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.210nMAssay Description:Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu...More data for this Ligand-Target Pair
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.220nMAssay Description:Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu...More data for this Ligand-Target Pair
Affinity DataIC50: 0.251nMAssay Description:Agonist activity at glucocorticoid receptor in rat INS-1 832/13 cells transfected with CCL2-promoter luciferase plasmid construct assessed as reducti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.316nMAssay Description:Agonist activity at glucocorticoid receptor in rat INS-1 832/13 cells transfected with CCL2-promoter luciferase plasmid construct assessed as reducti...More data for this Ligand-Target Pair
Target72 kDa type IV collagenase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.390nMAssay Description:Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f...More data for this Ligand-Target Pair
TargetCollagenase 3(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human MMP13 catalytic domain (amino acids 103 to 268) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preinc...More data for this Ligand-Target Pair
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.460nMAssay Description:Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu...More data for this Ligand-Target Pair
Target72 kDa type IV collagenase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.490nMAssay Description:Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f...More data for this Ligand-Target Pair
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Target72 kDa type IV collagenase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.530nMAssay Description:Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f...More data for this Ligand-Target Pair
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Target72 kDa type IV collagenase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.550nMAssay Description:Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f...More data for this Ligand-Target Pair
Target72 kDa type IV collagenase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.570nMAssay Description:Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f...More data for this Ligand-Target Pair
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.580nMAssay Description:Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu...More data for this Ligand-Target Pair
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Target72 kDa type IV collagenase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.631nMAssay Description:Agonist activity at glucocorticoid receptor in rat INS-1 832/13 cells transfected with CCL2-promoter luciferase plasmid construct assessed as reducti...More data for this Ligand-Target Pair
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Target72 kDa type IV collagenase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetCollagenase 3(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of human MMP13 catalytic domain (amino acids 103 to 268) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preinc...More data for this Ligand-Target Pair
Target72 kDa type IV collagenase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Target72 kDa type IV collagenase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetCollagenase 3(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibition of MMP13More data for this Ligand-Target Pair
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu...More data for this Ligand-Target Pair
Target72 kDa type IV collagenase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Target72 kDa type IV collagenase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu...More data for this Ligand-Target Pair
Target72 kDa type IV collagenase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f...More data for this Ligand-Target Pair
Target72 kDa type IV collagenase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Target72 kDa type IV collagenase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL