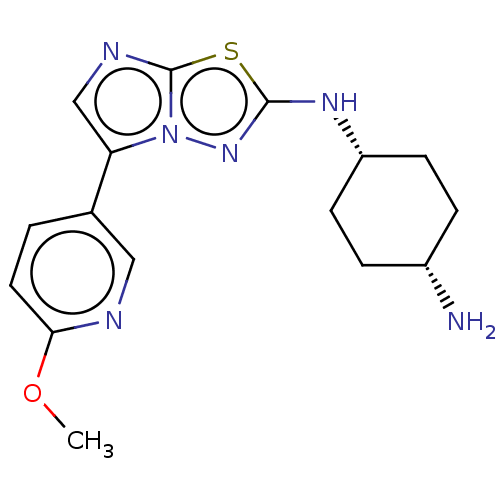
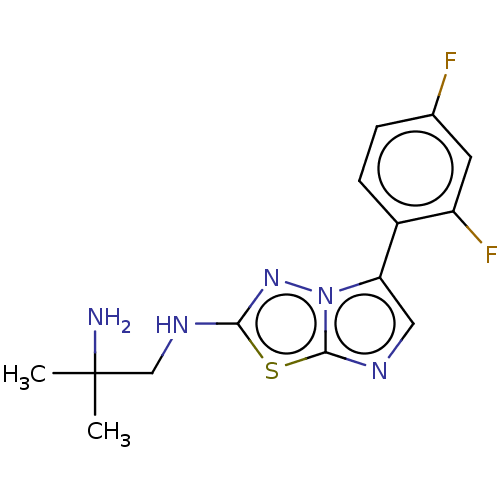
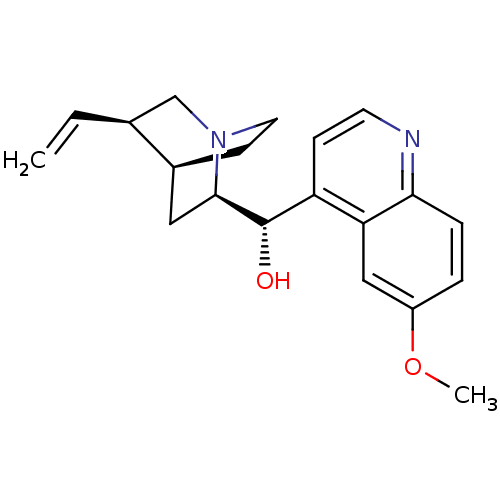
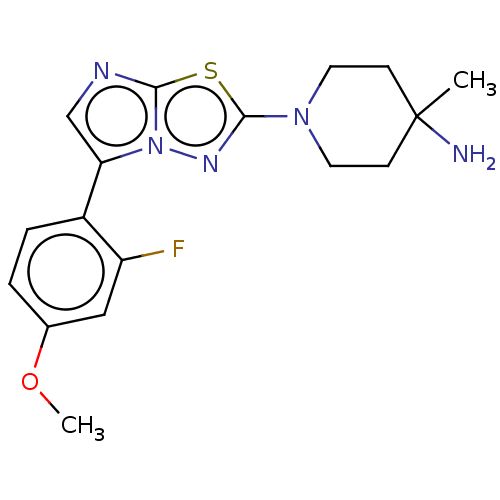
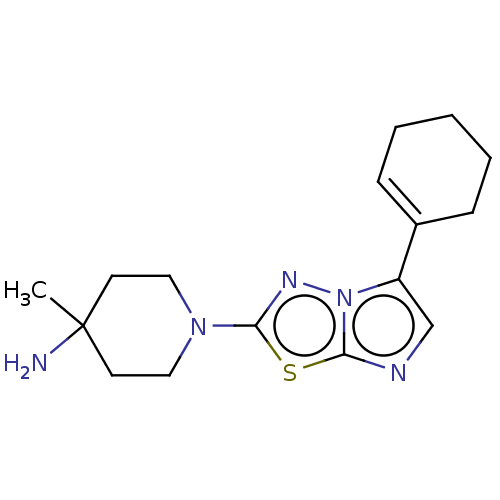
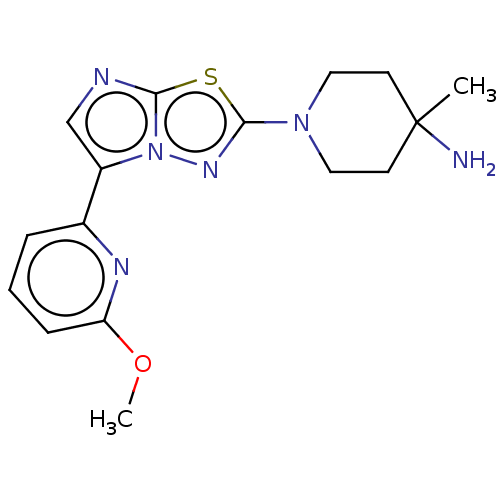
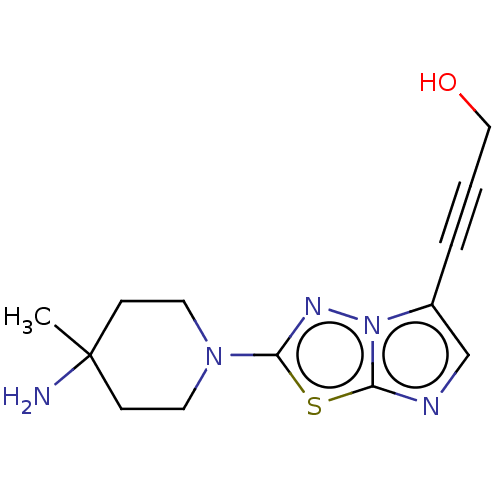
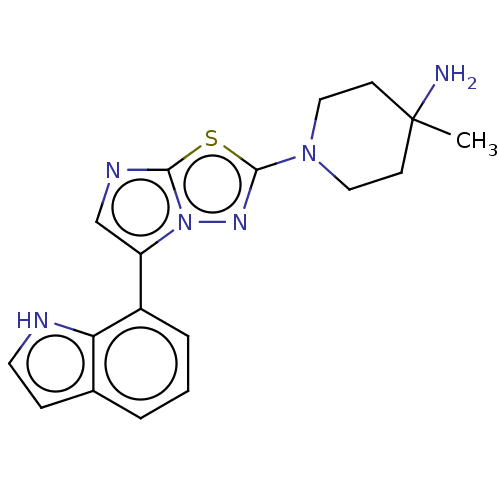
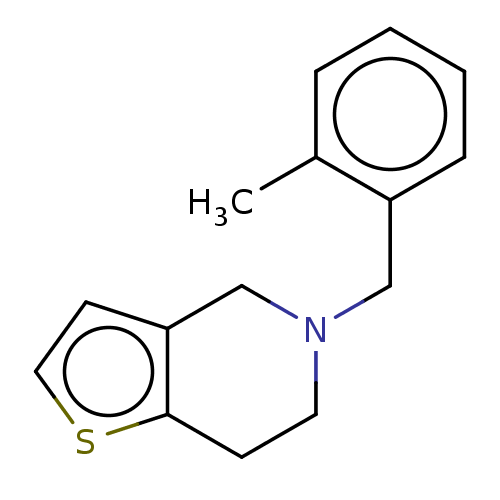
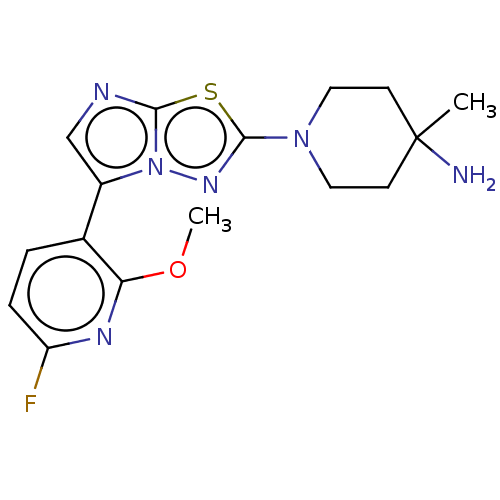
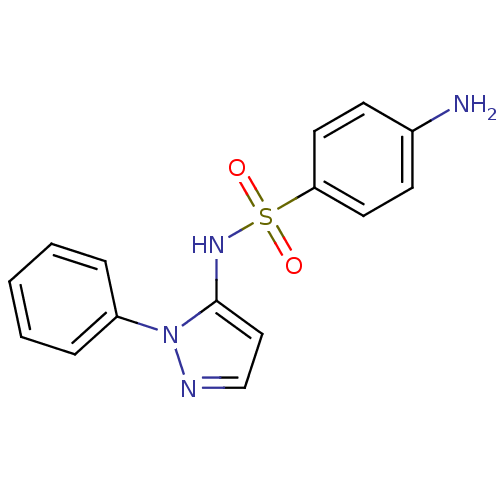
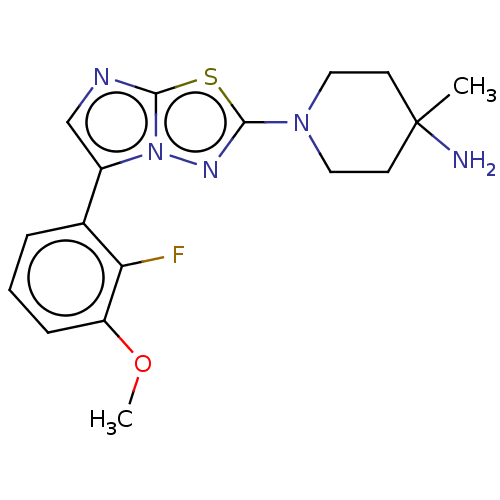
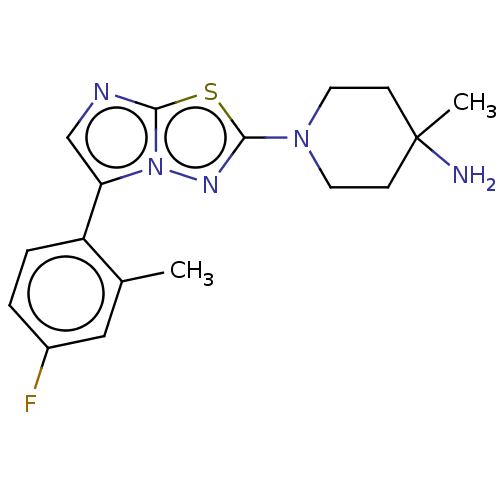
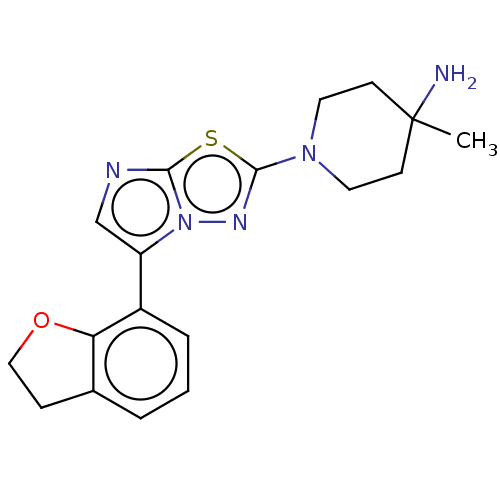
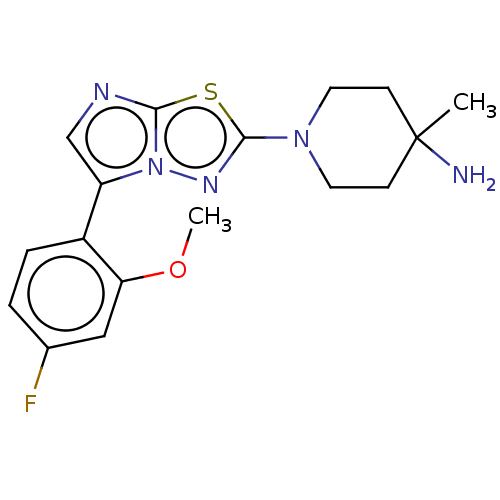
Affinity DataKi: 588nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.49E+3nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.08E+3nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 6.22E+3nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.12E+4nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 5.52E+4nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.37E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.44E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.59E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.80E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.99E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.99E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.33E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.34E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.66E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.86E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.98E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.45E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 5.51E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 5.95E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 7.33E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.61E+6nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.63E+6nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.93E+6nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.93E+6nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.30E+6nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.43E+6nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.52E+6nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.67E+6nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.18E+6nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 9.50E+7nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of CYP3A4 in human liver microsomes assessed as testosterone 6beta-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of CYP3A4 in human liver microsomes assessed as midazolam 1'-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibition of CYP2D6 in human liver microsomes assessed as dextromethorphan O-demethylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 45nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 78nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
Affinity DataIC50: 480nMAssay Description:Inhibition of CYP2C19 in human liver microsomes assessed as (S)-mephenytoin 4'-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 690nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
Affinity DataIC50: 720nMAssay Description:Inhibition of CYP2C9 in human liver microsomes assessed as tolbutamide methylhydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 770nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.28E+3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.33E+3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.74E+3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of hERG by Qpatch-clamp methodMore data for this Ligand-Target Pair

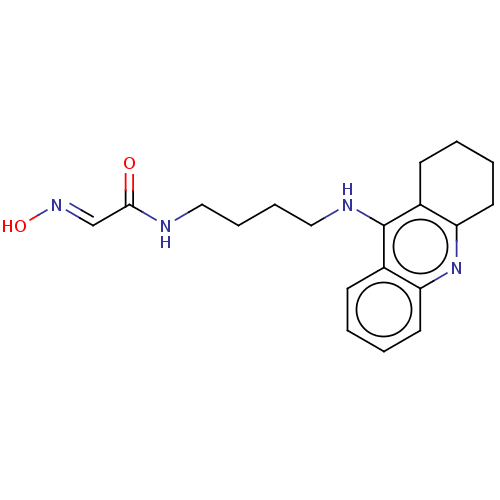
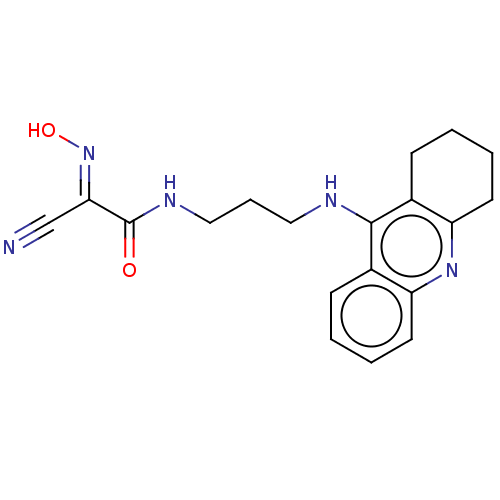


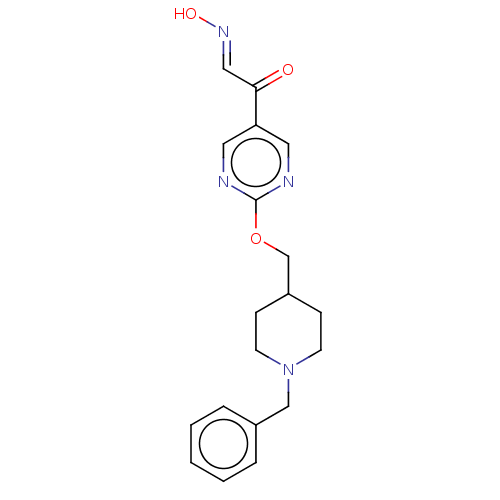

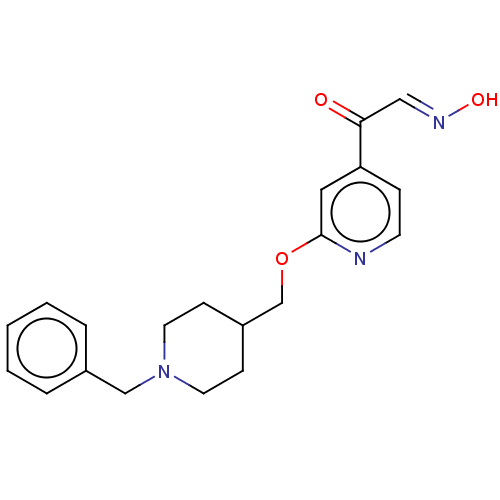
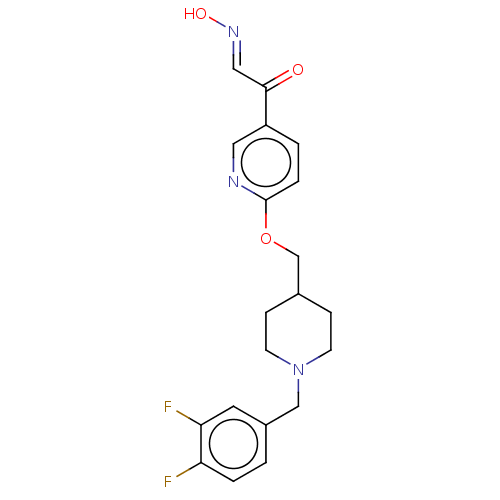

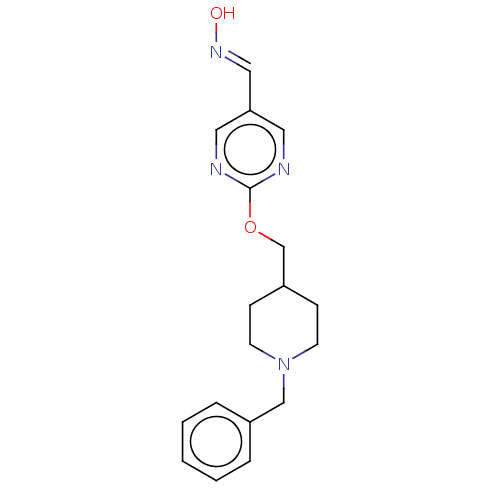
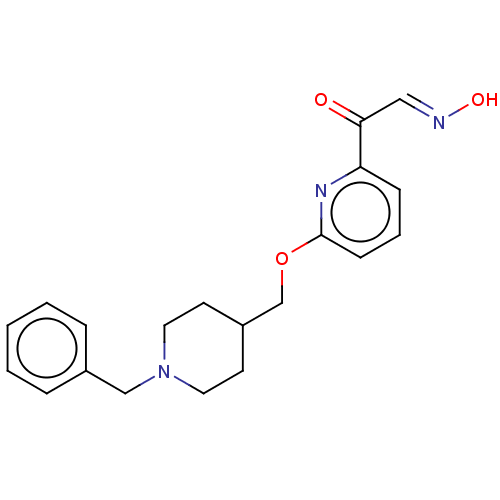

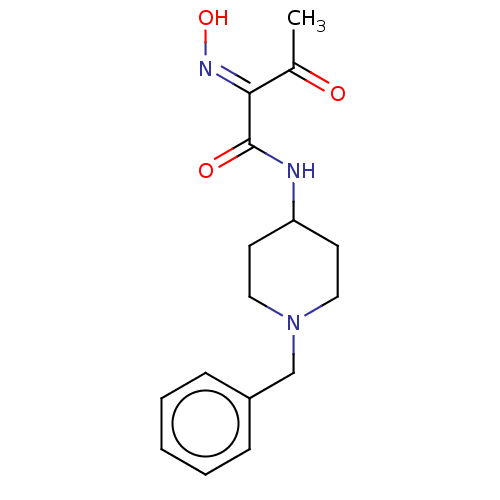
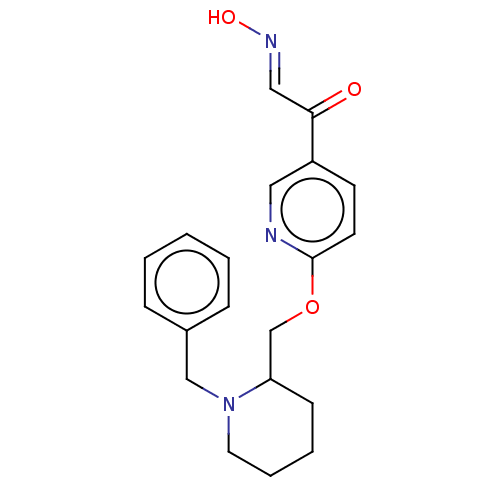


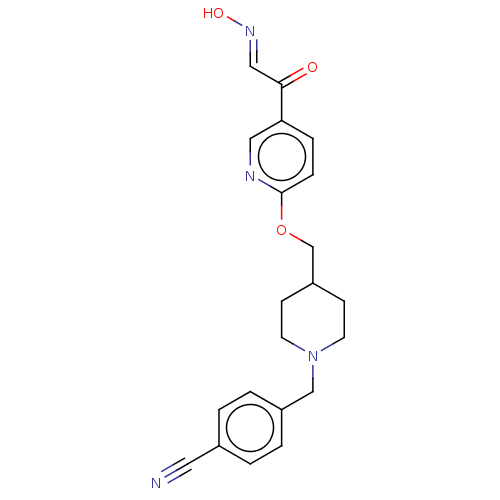

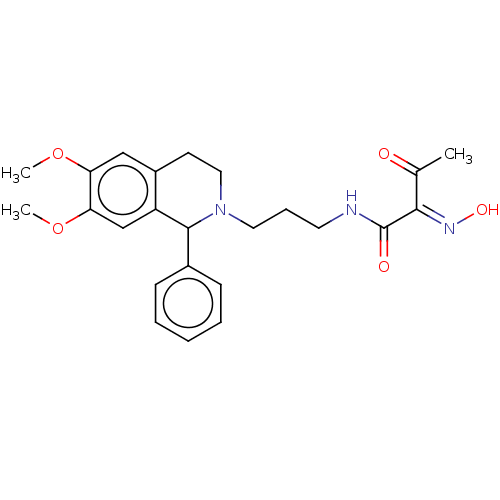
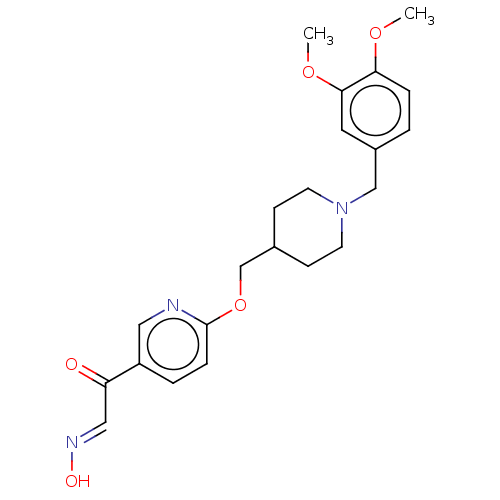

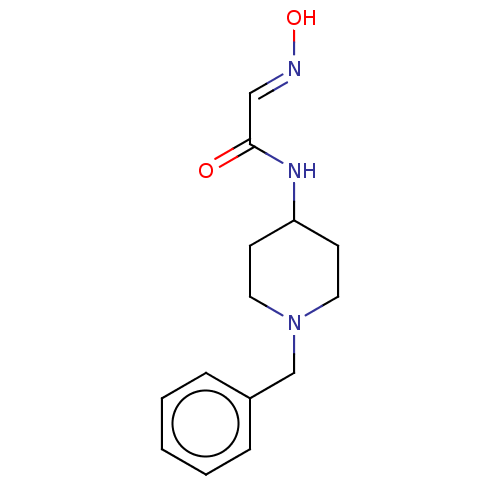
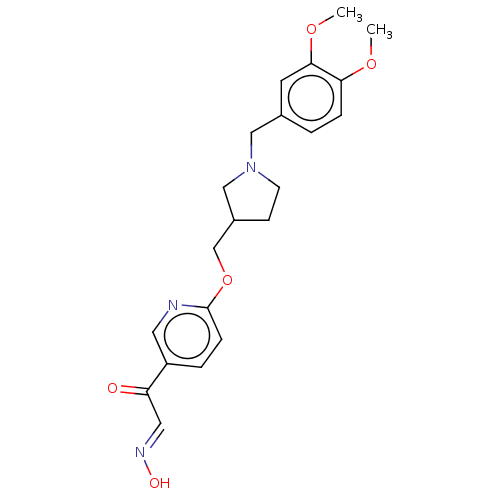
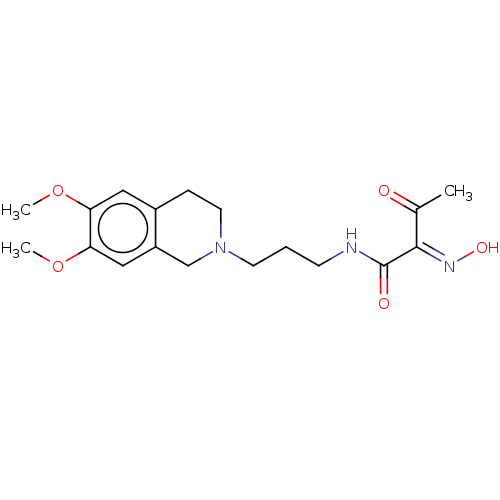
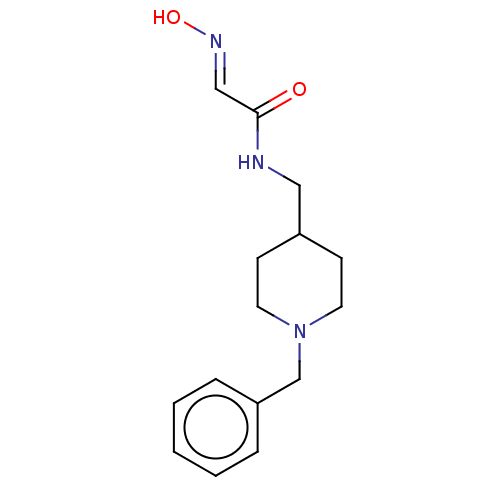
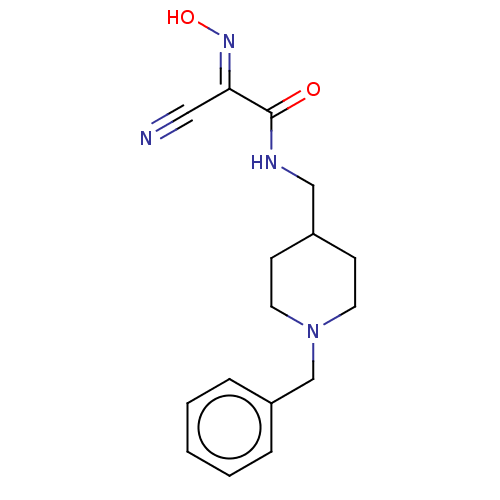
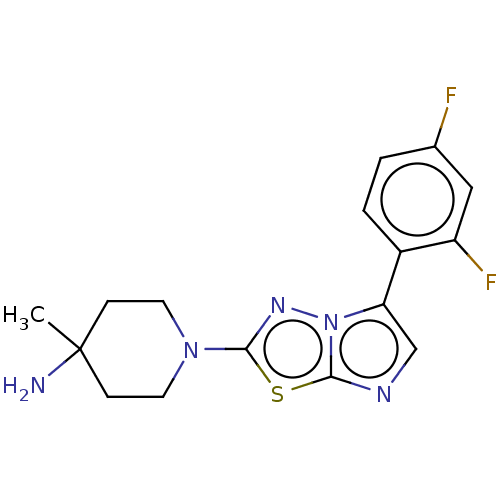
 3D Structure (crystal)
3D Structure (crystal)