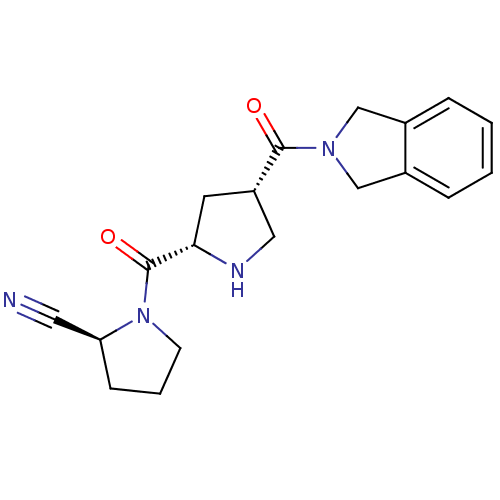
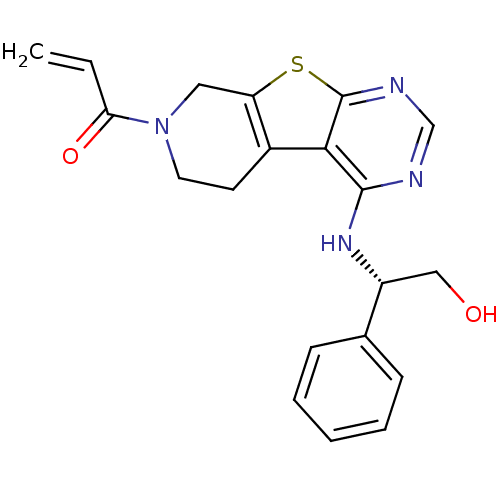
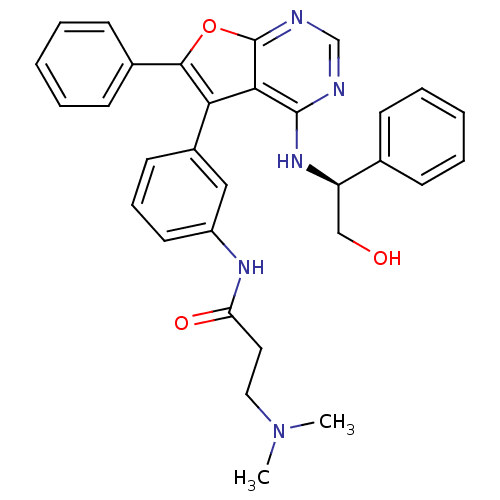
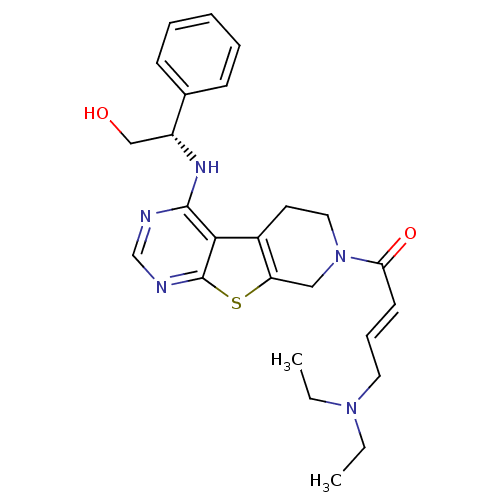
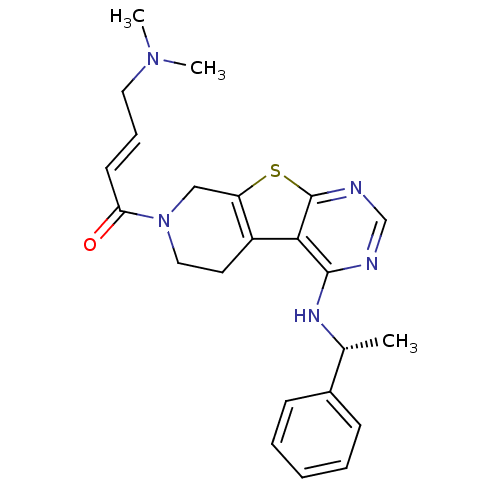
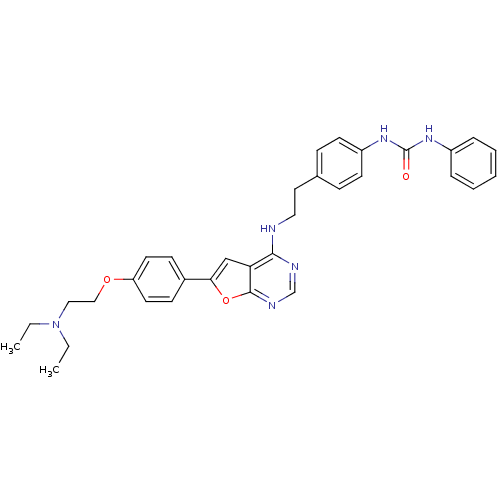
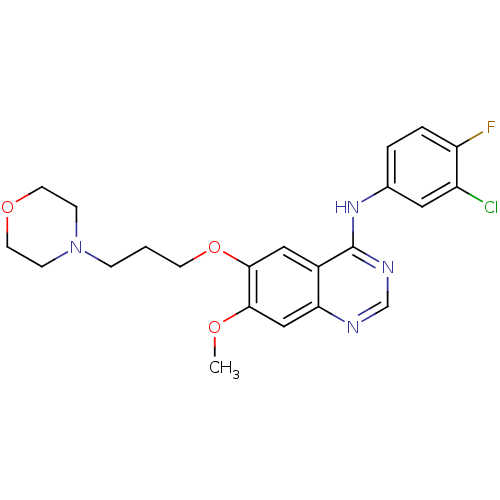
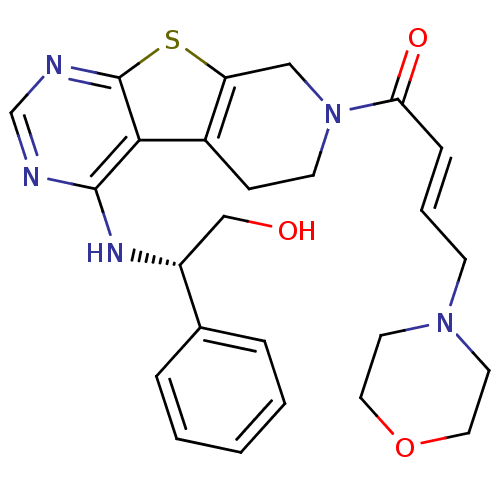
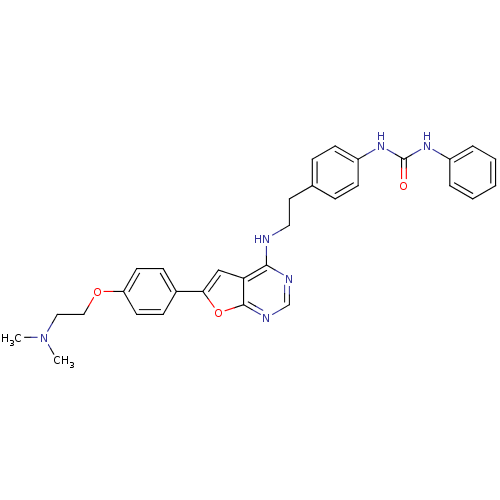
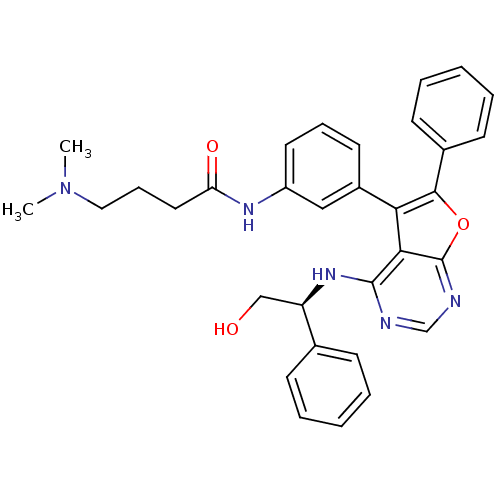
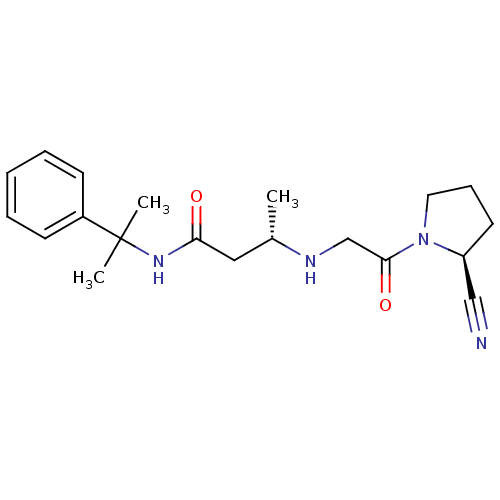
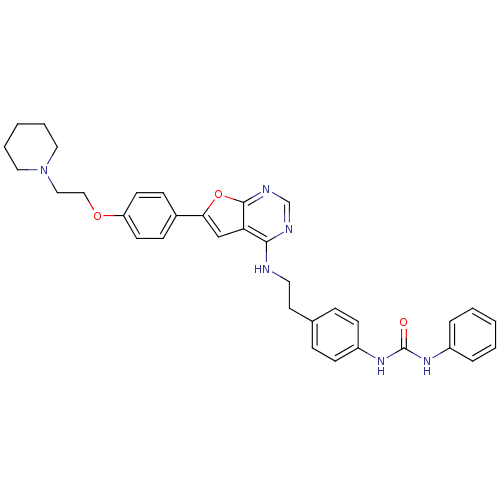
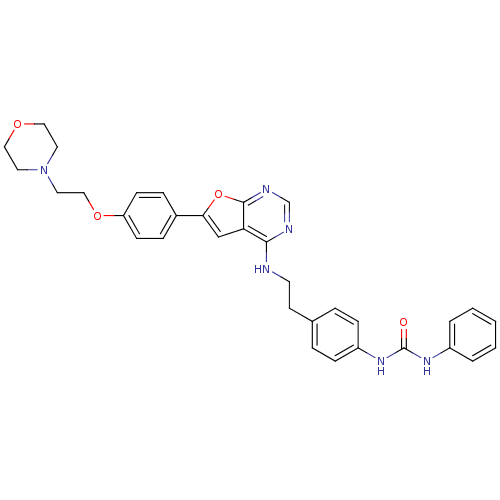
Affinity DataKi: 0.600nMAssay Description:Inhibition of Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Inhibition of Aurora B kinaseMore data for this Ligand-Target Pair
Affinity DataKi: 4.60nMAssay Description:Inhibition of Aurora C kinaseMore data for this Ligand-Target Pair
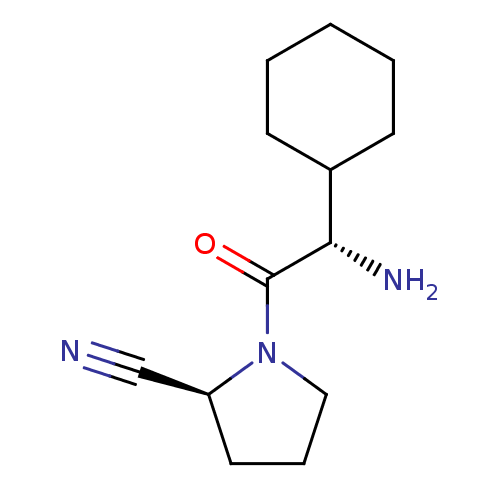
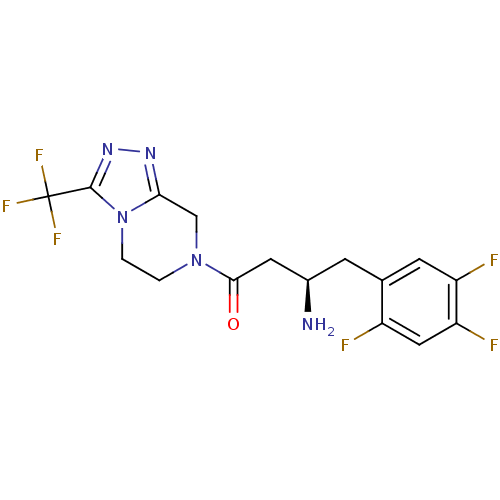
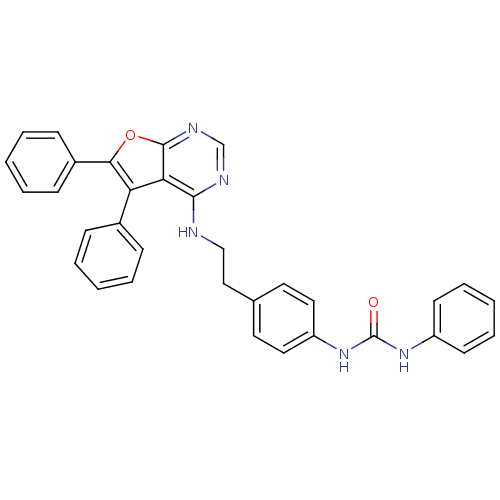
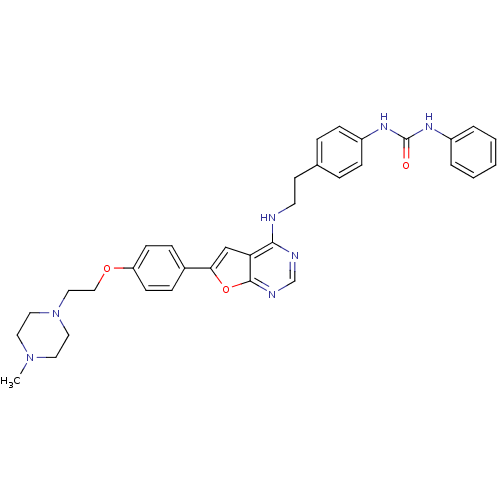
Affinity DataKi: 13nMAssay Description:Inhibition of Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataKi: 61nMAssay Description:Inhibition of Aurora C kinaseMore data for this Ligand-Target Pair
Affinity DataKi: 79nMAssay Description:Inhibition of Aurora B kinaseMore data for this Ligand-Target Pair
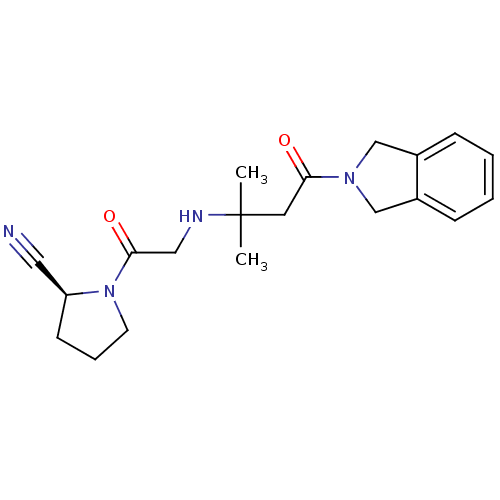
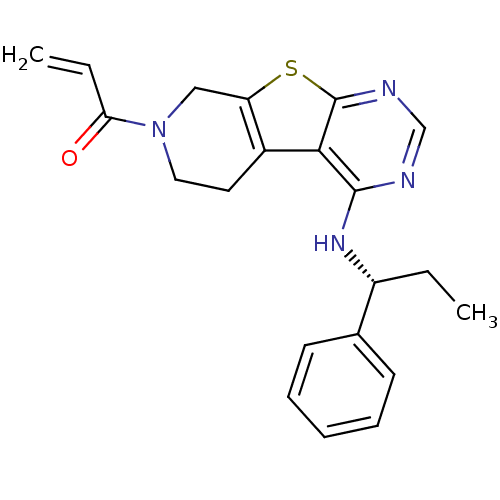
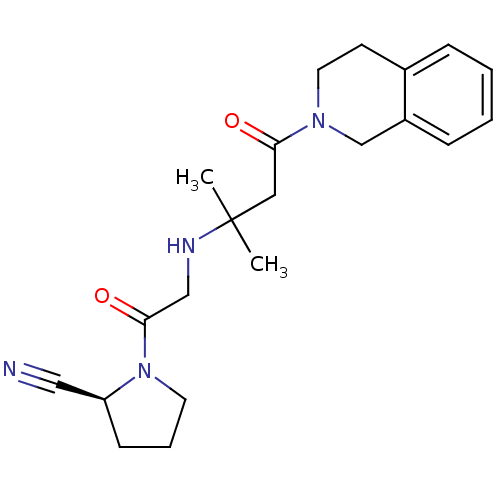
Affinity DataIC50: 1.70nMpH: 8.0 T: 2°CAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair
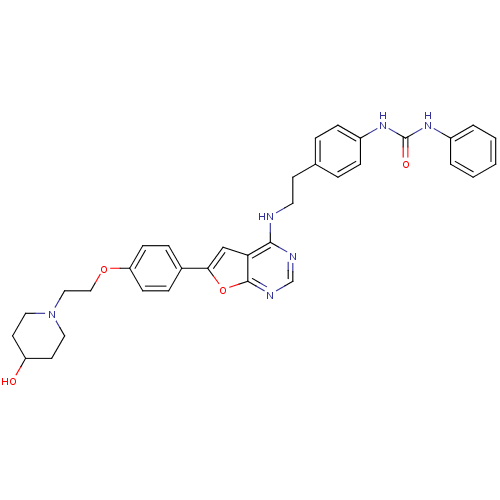
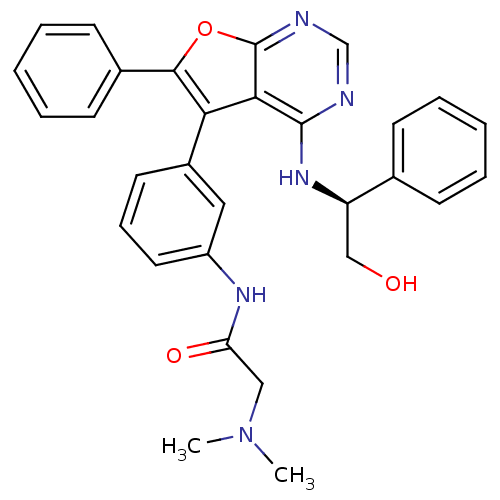
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
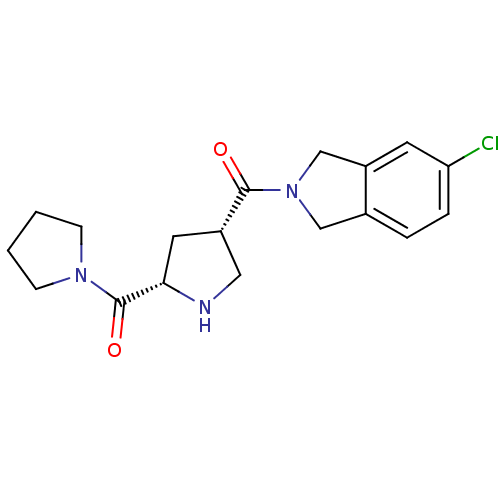
Affinity DataIC50: 7nMAssay Description:Inhibition of GST-tagged EGFR expressed in Escherichia coliChecked by AuthorMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of human wild type GST-tagged EGFR kinase domain expressed in Sf9 cells by luminescence assayMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of GST-tagged EGFR expressed in Escherichia coliChecked by AuthorMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 12nMpH: 8.0 T: 2°CAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMpH: 8.0 T: 2°CAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of Aurora kinase B (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibition of GST-tagged Aurora kinase A catalytic domain (123 to 401 amino acids) (unknown origin) expressed in sf9 cells using tetra(LRRWSLG) as su...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of GST-tagged EGFR expressed in Escherichia coliChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of Aurora AChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of GST-tagged Aurora kinase A catalytic domain (123 to 401 amino acids) (unknown origin) expressed in sf9 cells using tetra(LRRWSLG) as su...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of Aurora kinase A (unknown origin)More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibition of Aurora kinase A (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 29nMAssay Description:Inhibition of human wild type GST-tagged EGFR kinase domain expressed in Sf9 cells by luminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of Aurora kinase B (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 8.0 T: 2°CAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 33nMAssay Description:Inhibition of human wild type GST-tagged EGFR kinase domain expressed in Sf9 cells by luminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 33nMAssay Description:Inhibition of Aurora kinase A T288D mutant expressed in Escherichia coli BL21 DE3More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Inhibition of human wild type GST-tagged EGFR kinase domain expressed in Sf9 cells by luminescence assayMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of human wild type GST-tagged EGFR kinase domain expressed in Sf9 cells by luminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Inhibition of Aurora kinase A (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:In vitro inhibition of DPP-4More data for this Ligand-Target Pair
Affinity DataIC50: 43nMAssay Description:Inhibition of Aurora AChecked by AuthorMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 48nMAssay Description:Inhibition of human wild type GST-tagged EGFR kinase domain expressed in Sf9 cells by luminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 49nMAssay Description:Inhibition of GST-tagged Aurora kinase A catalytic domain (123 to 401 amino acids) (unknown origin) expressed in sf9 cells using tetra(LRRWSLG) as su...More data for this Ligand-Target Pair
Affinity DataIC50: 49nMAssay Description:Inhibition of GST-tagged Aurora kinase A catalytic domain (123 to 401 amino acids) (unknown origin) expressed in sf9 cells using tetra(LRRWSLG) as su...More data for this Ligand-Target Pair
Affinity DataIC50: 49nMAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMpH: 8.0 T: 2°CAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of GST-tagged Aurora kinase A catalytic domain (123 to 401 amino acids) (unknown origin) expressed in sf9 cells using tetra(LRRWSLG) as su...More data for this Ligand-Target Pair
Affinity DataIC50: 51nMpH: 8.0 T: 2°CAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair
Affinity DataIC50: 51nMpH: 8.0 T: 2°CAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair
Affinity DataIC50: 53nMpH: 8.0 T: 2°CAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)