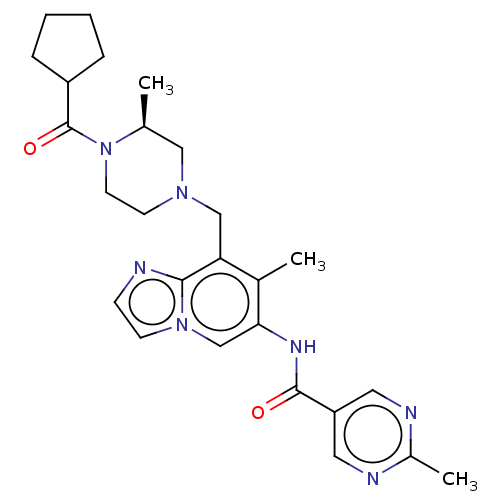
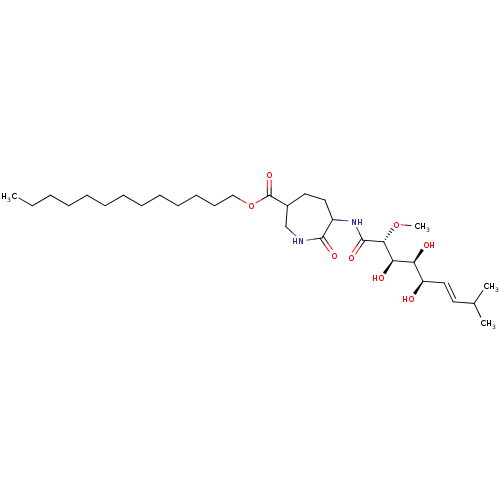
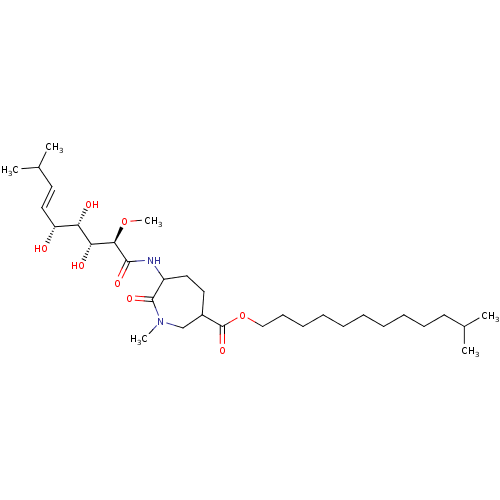
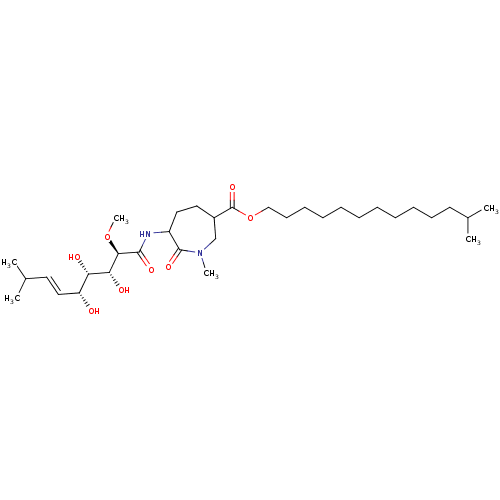
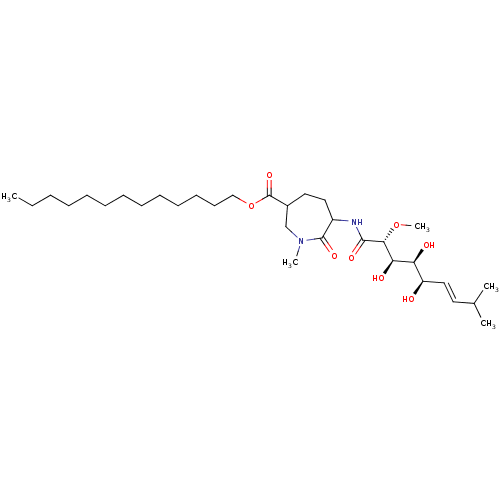
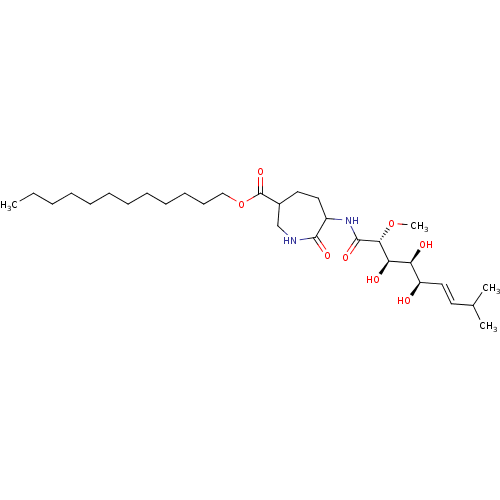
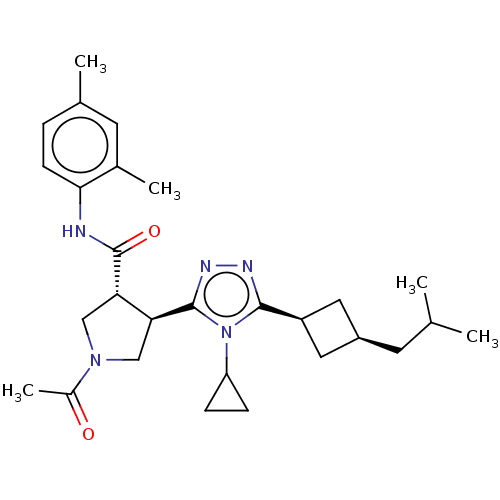
Affinity DataKi: 3.70nMAssay Description:The calpain inhibitory activity(I 50) was measured as ability to enter the platelet to inhibit calpain after cell lysis(assay 2)More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The ability of compound to inhibit calpain in a preparation of lysed platelets was measured with a caseinolytic assay(assay 1)More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compound was evaluated for the inhibition of Escherichia coli Thymidylate SynthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibitory activity against Escherichia coli dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The ability of compound to inhibit calpain in a preparation of lysed platelets was measured with a caseinolytic assay(assay 1)More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Compound was evaluated for the inhibition of Escherichia coli Thymidylate SynthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Compound was evaluated for the inhibition of Escherichia coli Thymidylate SynthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:The ability of compound to inhibit calpain in a preparation of lysed platelets was measured with a caseinolytic assay(assay 1)More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:The ability of compound to inhibit calpain in a preparation of lysed platelets was measured with a caseinolytic assay(assay 1)More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:The ability of compound to inhibit calpain in a preparation of lysed platelets was measured with a caseinolytic assay(assay 1)More data for this Ligand-Target Pair
Affinity DataIC50: 35nMAssay Description:Compound was evaluated for the inhibition of Escherichia coli Thymidylate SynthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 221nMAssay Description:The calpain inhibitory activity(I 50) was measured as ability to enter the platelet to inhibit calpain after cell lysis(assay 2)More data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro.More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic(Homo sapiens (Human))
Guizhou Medcial University
Curated by ChEMBL
Guizhou Medcial University
Curated by ChEMBL
Affinity DataIC50: 5.18E+3nMAssay Description:Inhibition of IDH1 R132C mutant (unknown origin) by enzymatic assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.40E+3nMAssay Description:The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 1.05E+4nMAssay Description:The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 1.79E+4nMAssay Description:The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 2.68E+4nMAssay Description:The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 2.93E+4nMAssay Description:The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 3.71E+4nMAssay Description:The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 4.02E+4nMAssay Description:The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro.More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic(Homo sapiens (Human))
Guizhou Medcial University
Curated by ChEMBL
Guizhou Medcial University
Curated by ChEMBL
Affinity DataIC50: 7.69E+4nMAssay Description:Inhibition of IDH1 R132H mutant (unknown origin) by enzymatic assayMore data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic(Homo sapiens (Human))
Guizhou Medcial University
Curated by ChEMBL
Guizhou Medcial University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of IDH1 (unknown origin) by enzymatic assayMore data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic(Homo sapiens (Human))
Guizhou Medcial University
Curated by ChEMBL
Guizhou Medcial University
Curated by ChEMBL
Affinity DataKd: 1.28E+4nMAssay Description:Binding affinity to IDH1 R132H mutant (unknown origin) by surface plasmon resonance assayMore data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic(Homo sapiens (Human))
Guizhou Medcial University
Curated by ChEMBL
Guizhou Medcial University
Curated by ChEMBL
Affinity DataKd: >5.00E+4nMAssay Description:Binding affinity to IDH1 (unknown origin) by surface plasmon resonance assayMore data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic(Homo sapiens (Human))
Guizhou Medcial University
Curated by ChEMBL
Guizhou Medcial University
Curated by ChEMBL
Affinity DataKd: 2.81E+3nMAssay Description:Binding affinity to IDH1 R132C mutant (unknown origin) by surface plasmon resonance assayMore data for this Ligand-Target Pair
Affinity DataEC50: 160nMAssay Description:Compound was evaluated for the inhibition of Escherichia coli Thymidylate SynthaseMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)