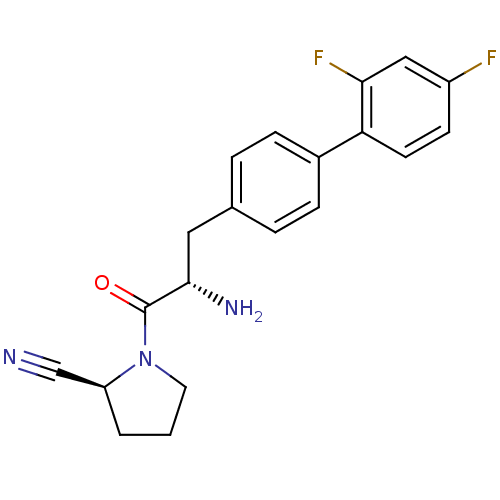
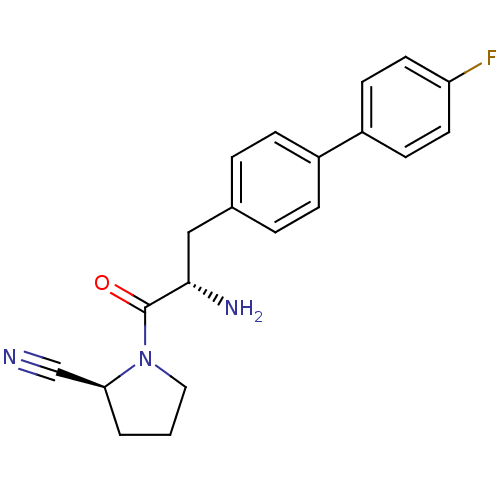
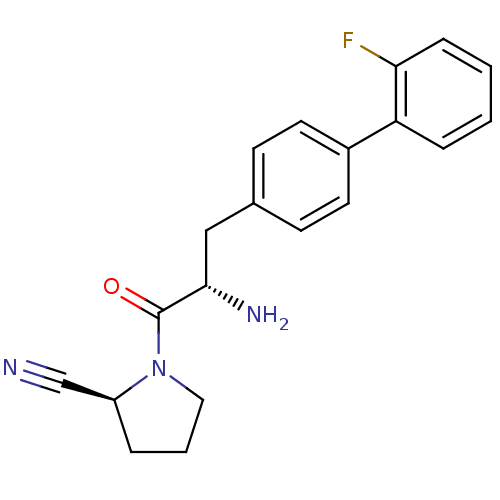
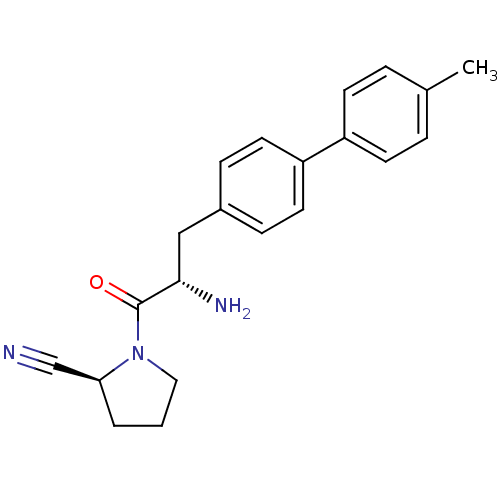
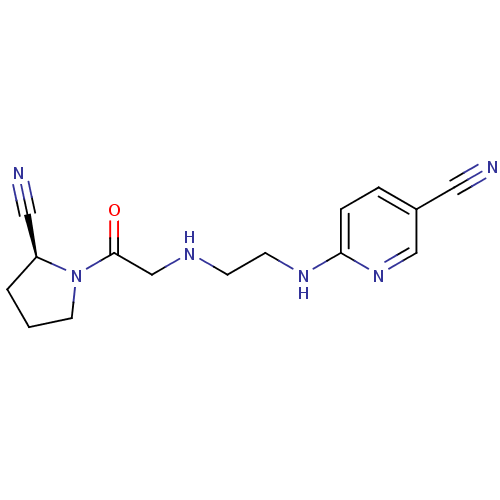
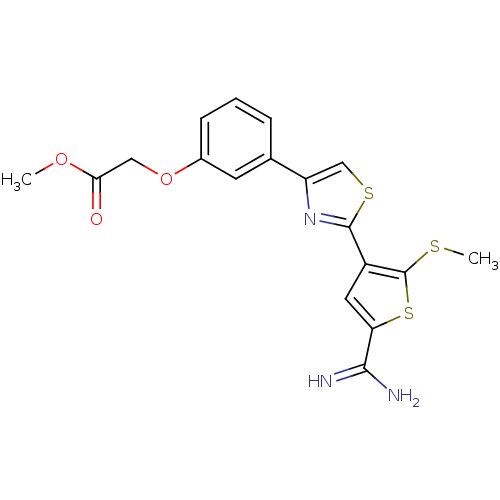
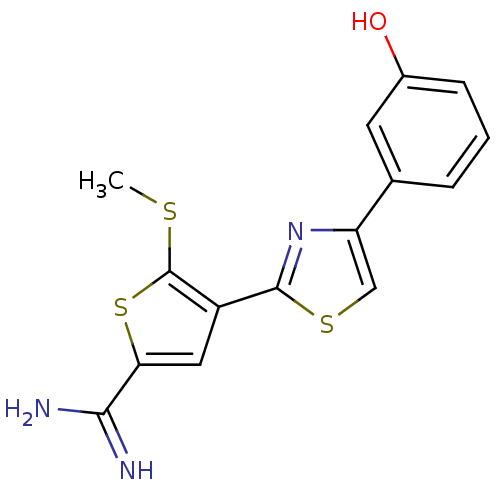
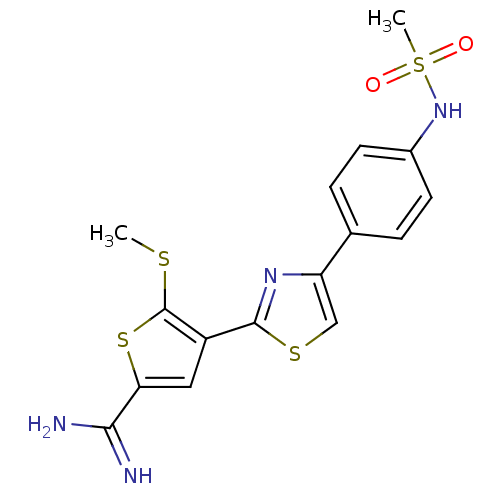
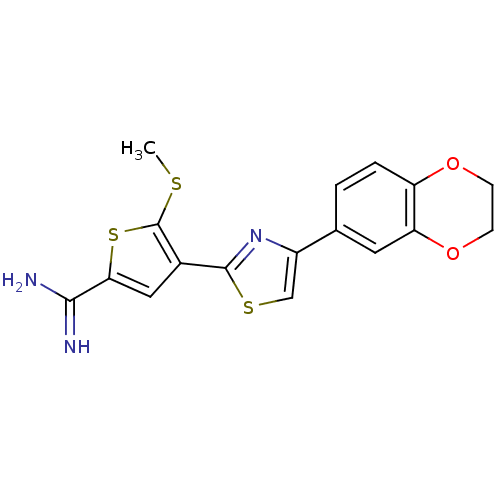
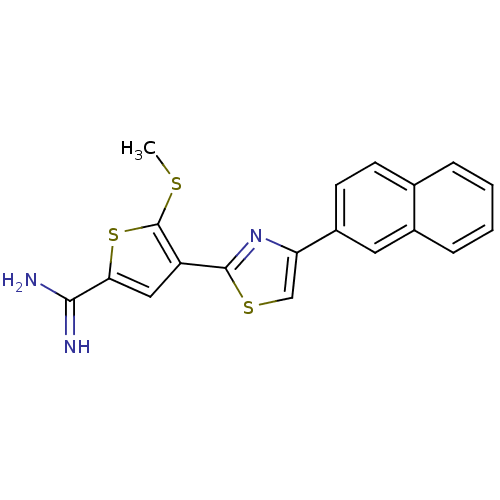
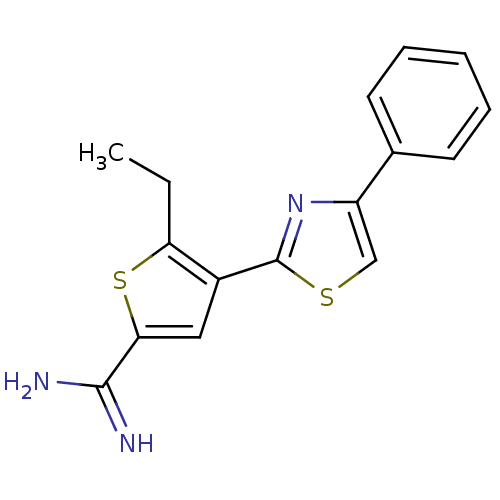
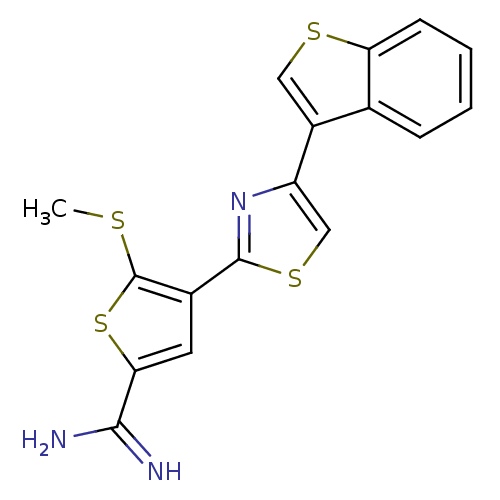
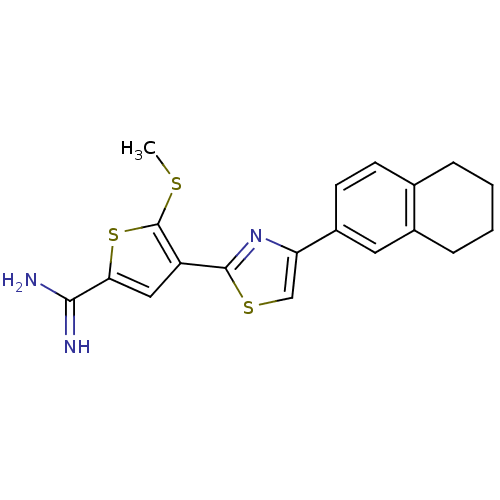
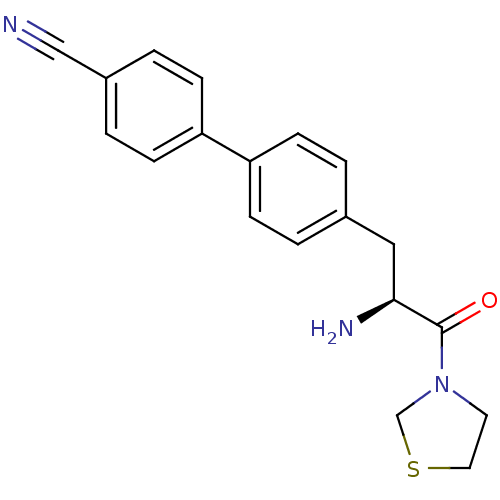
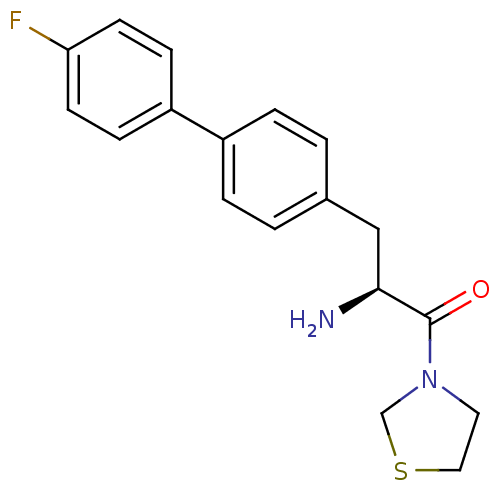
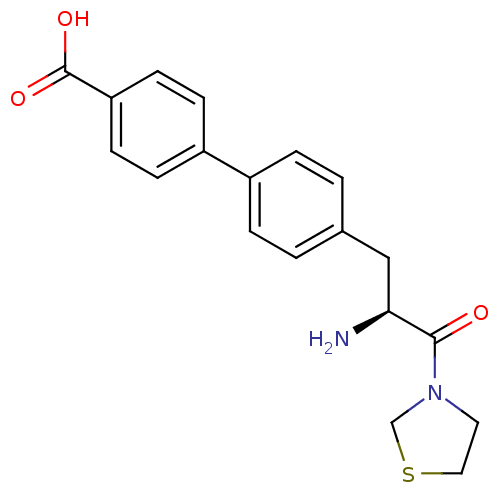
Affinity DataKi: 2.20nM ΔG°: -49.4kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
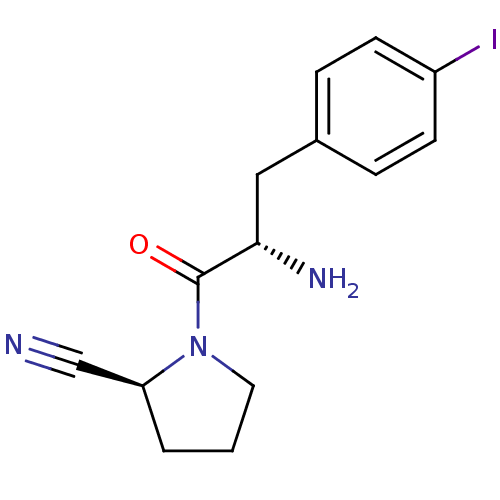
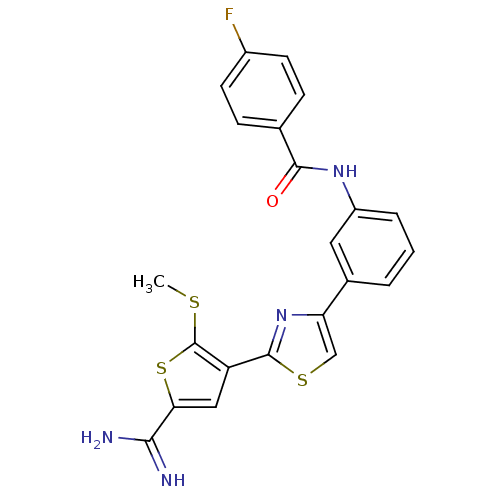
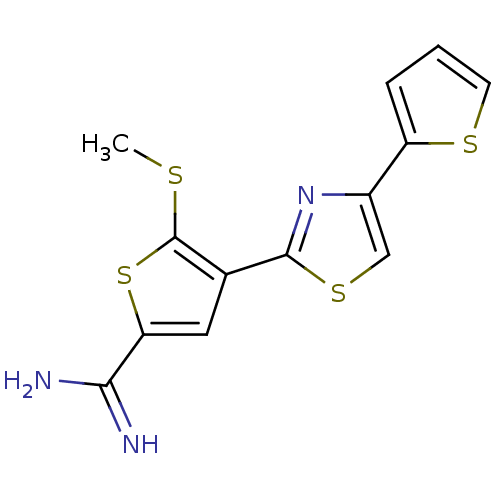
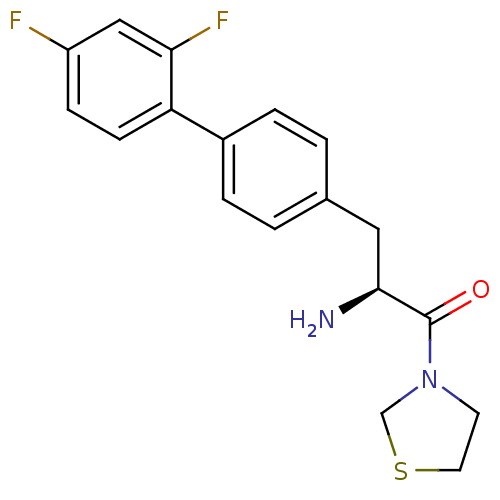
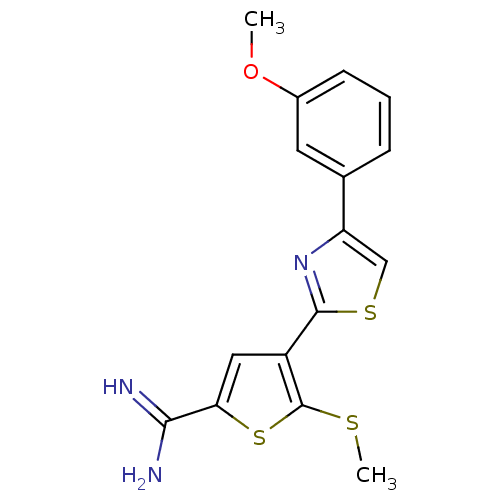
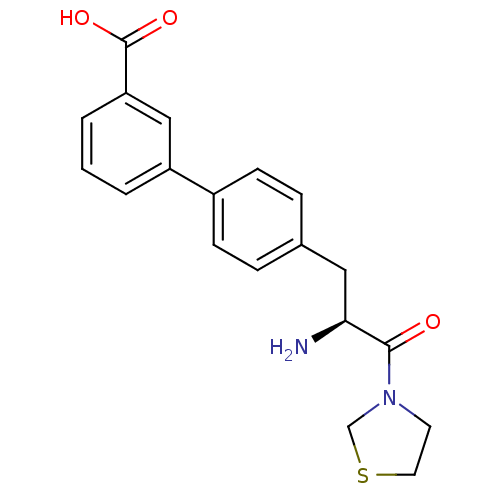
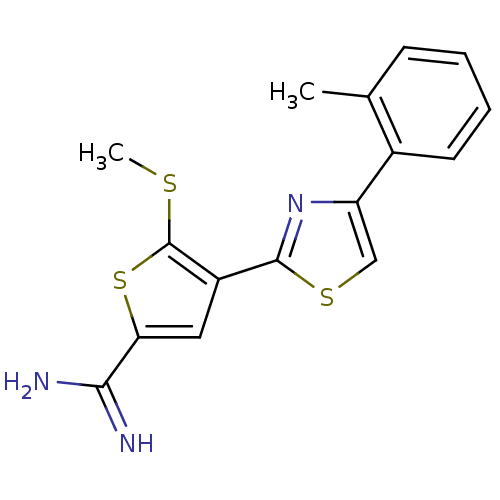
Affinity DataKi: 3.10nM ΔG°: -48.6kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
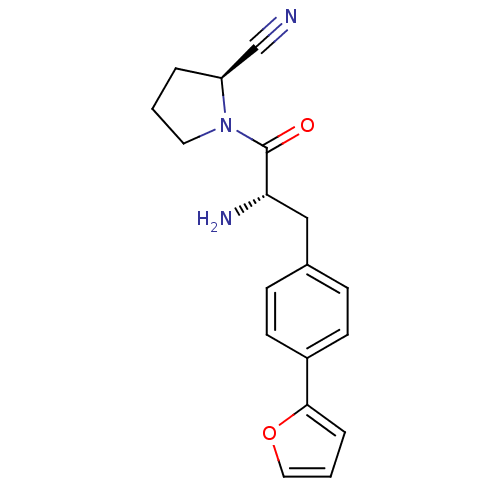
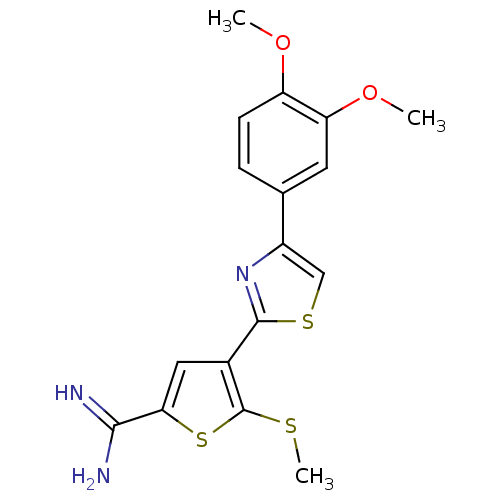
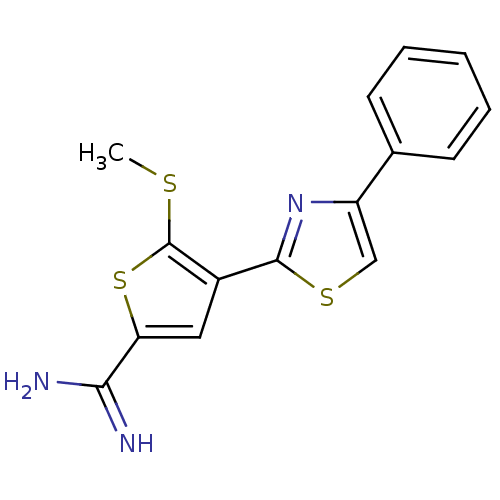
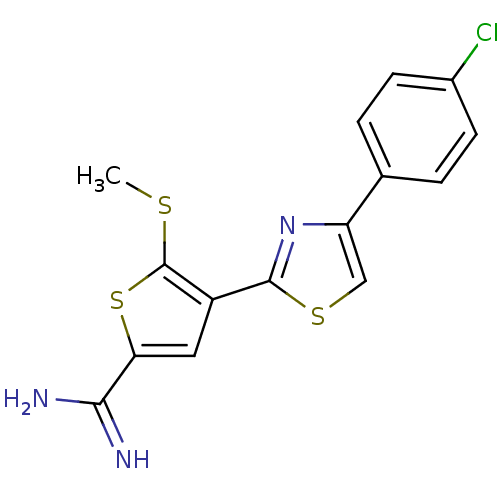
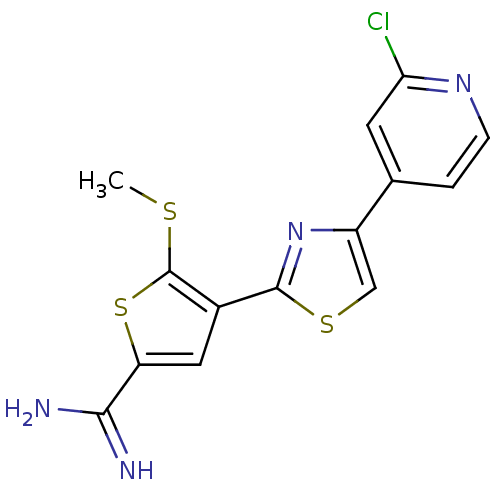
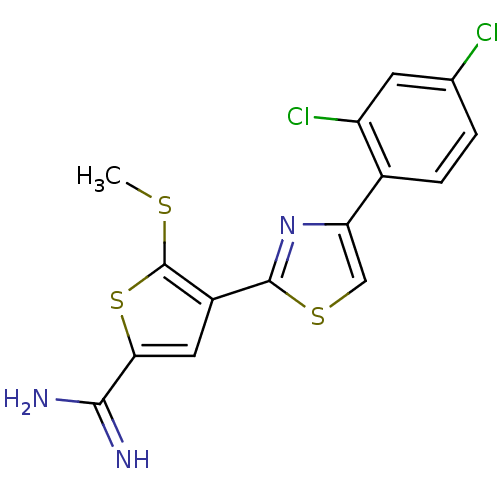
Affinity DataKi: 5.30nM ΔG°: -47.2kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
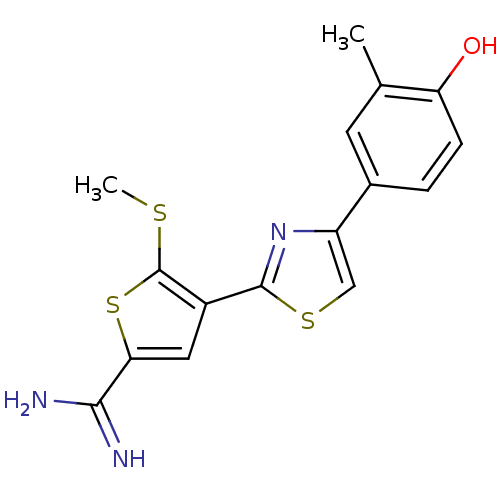
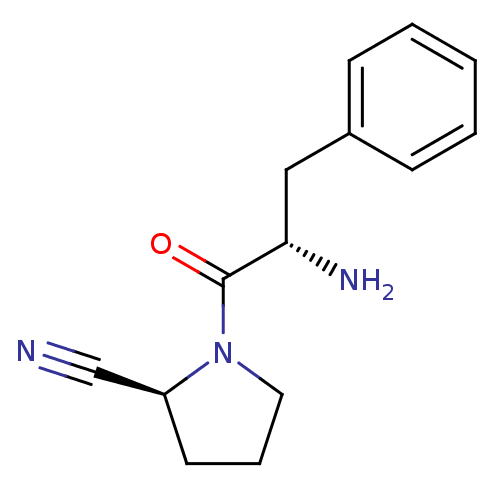
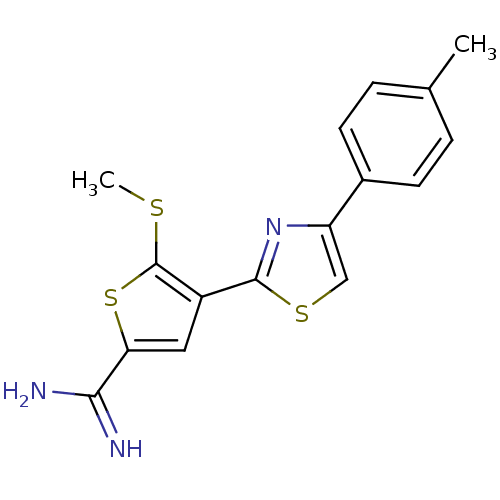
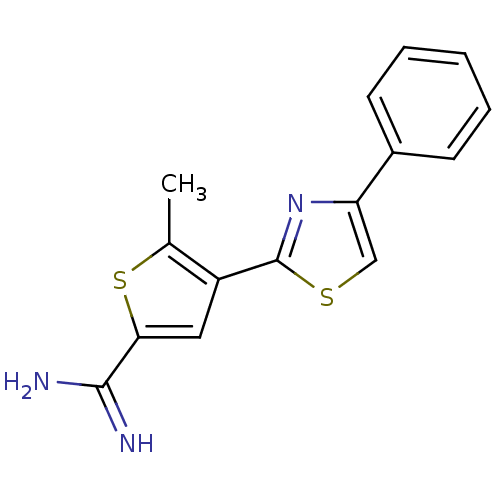
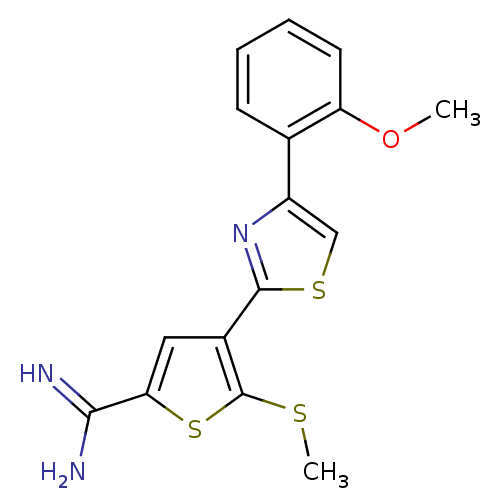
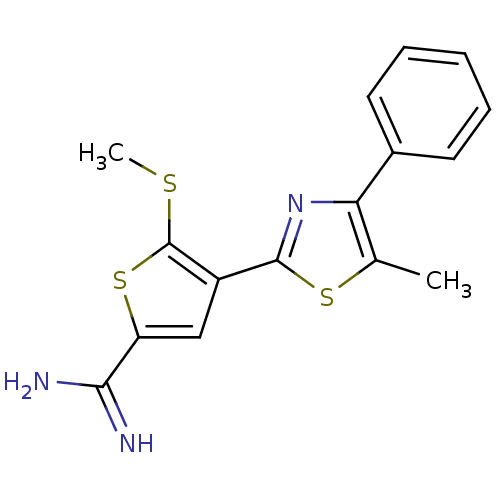
Affinity DataKi: 13nM ΔG°: -45.0kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 20nM ΔG°: -43.9kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 26nM ΔG°: -43.3kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 27nM ΔG°: -43.2kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 34nM ΔG°: -42.6kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 36nM ΔG°: -42.5kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
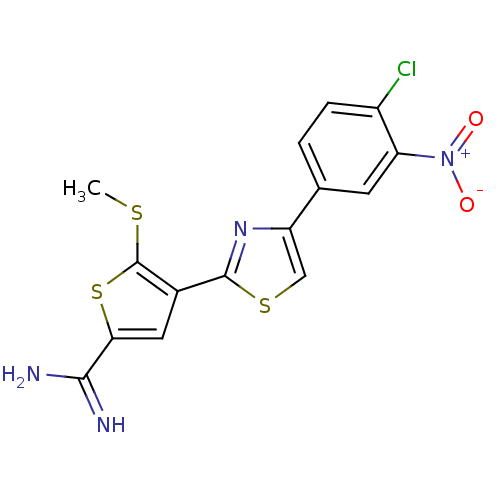
Affinity DataKi: 44nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
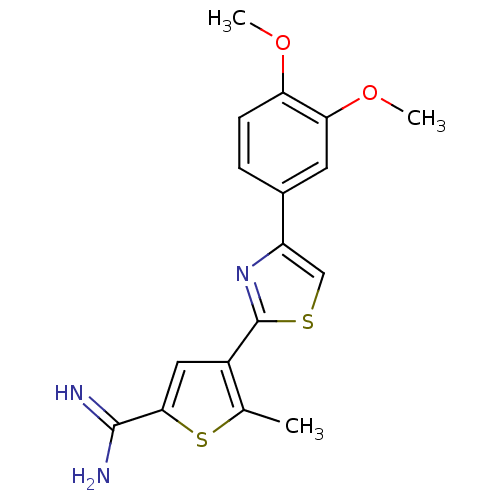
Affinity DataKi: 44nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 47nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 58nMAssay Description:Inhibition of Human kidney cell urokinaseMore data for this Ligand-Target Pair
Affinity DataKi: 63nM ΔG°: -41.1kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 86nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 89nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 90nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 91nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 94nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 94nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
Affinity DataKi: 96nM ΔG°: -40.1kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 99nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 100nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 101nMAssay Description:Inhibition of Human kidney cell urokinaseMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 102nMAssay Description:Inhibition of Human kidney cell urokinaseMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 103nMAssay Description:Inhibition of Human kidney cell urokinaseMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 108nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 115nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 120nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 130nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 138nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 138nMAssay Description:Inhibition of Human kidney cell urokinaseMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 141nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 145nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 152nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 154nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
Affinity DataKi: 160nM ΔG°: -38.8kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 161nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 164nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
Affinity DataKi: 166nM ΔG°: -38.7kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 169nMAssay Description:Inhibition of Human kidney cell urokinaseMore data for this Ligand-Target Pair
Affinity DataKi: 170nM ΔG°: -38.6kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 178nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 200nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 270nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 272nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 277nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 287nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 305nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
Affinity DataKi: 310nM ΔG°: -37.1kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair