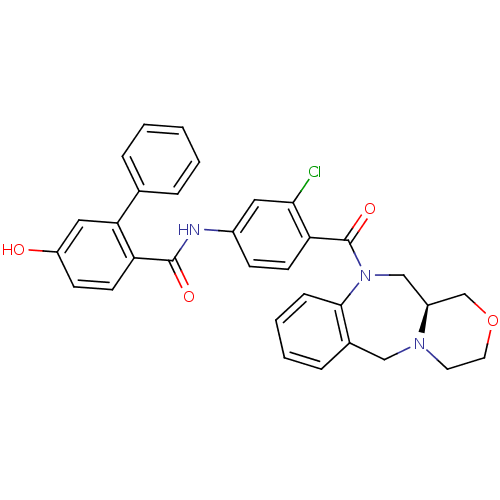
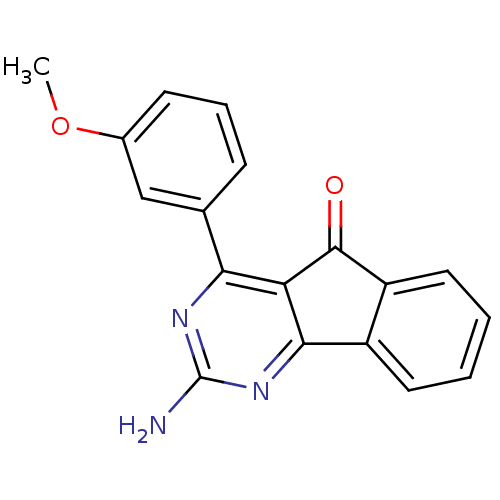
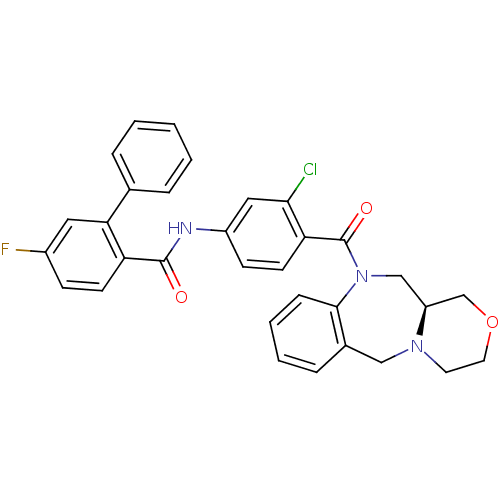
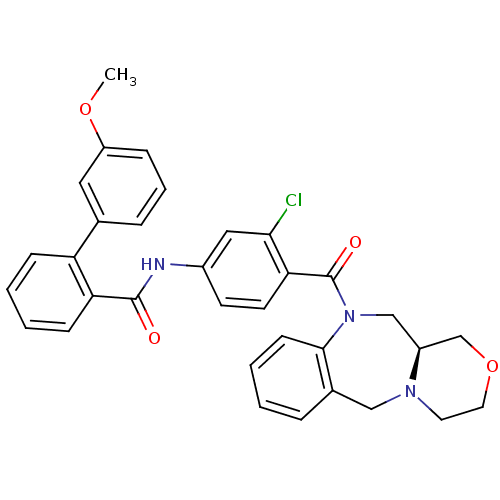
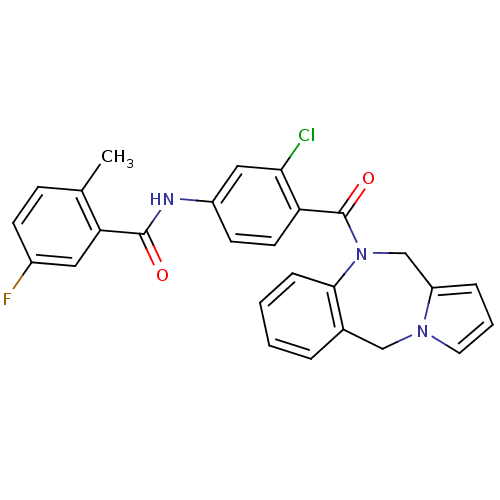
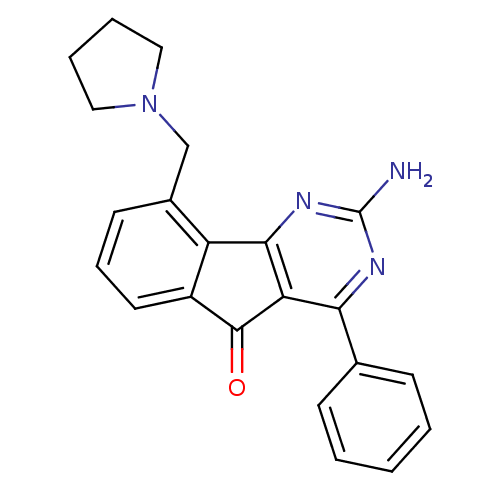
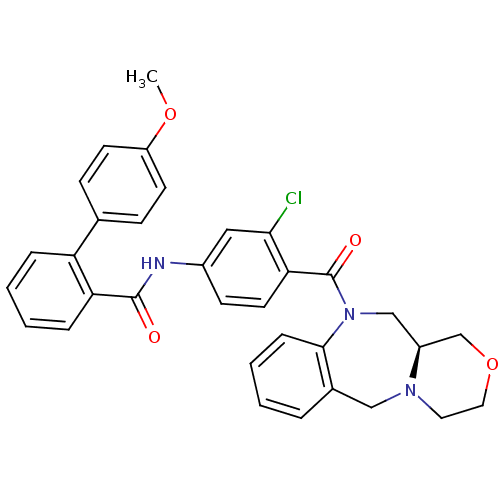
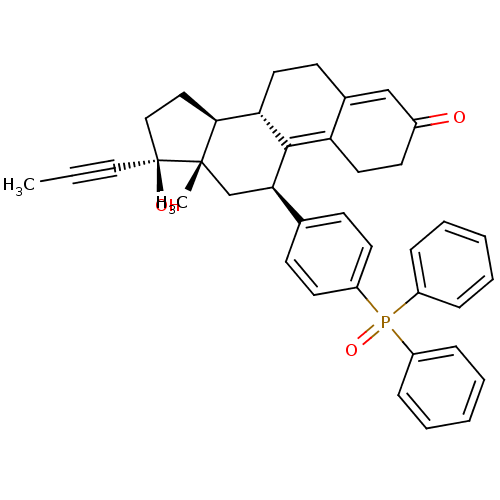
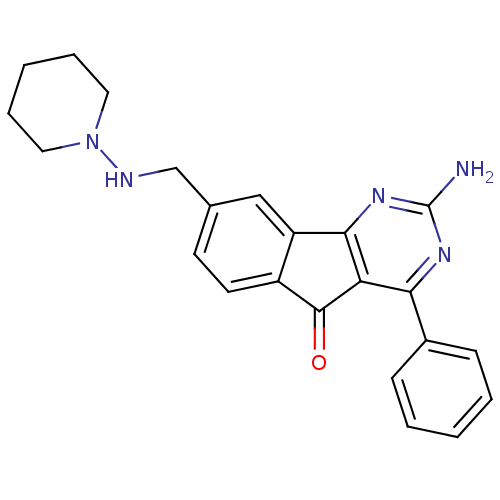
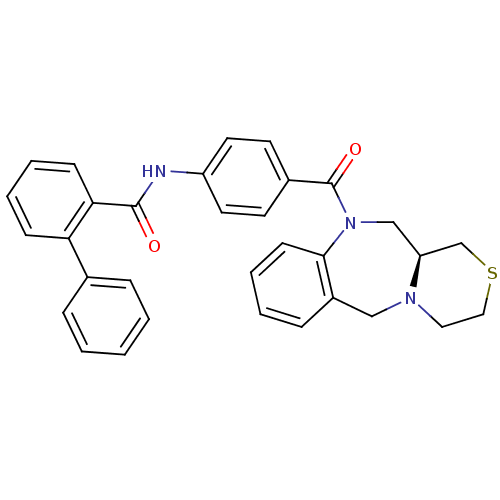
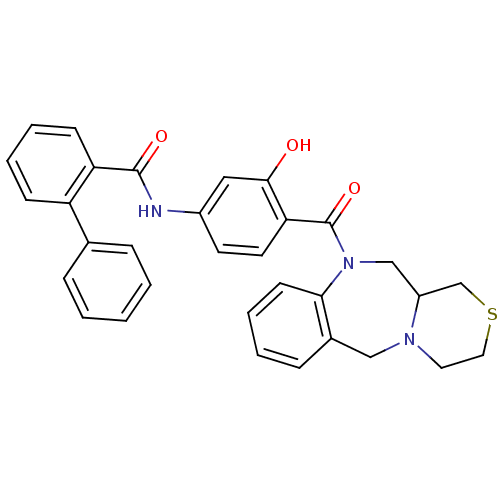
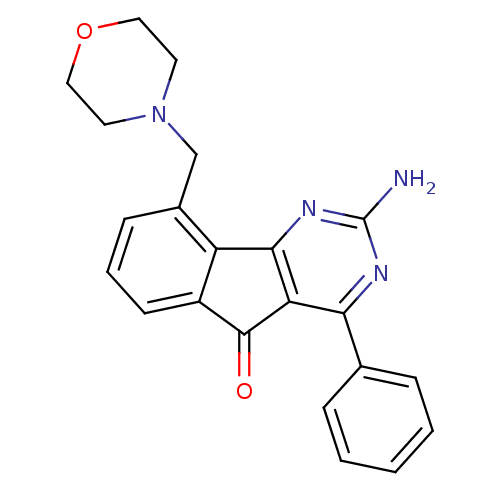
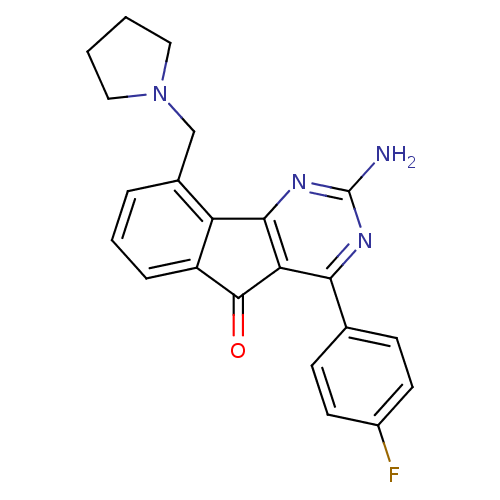
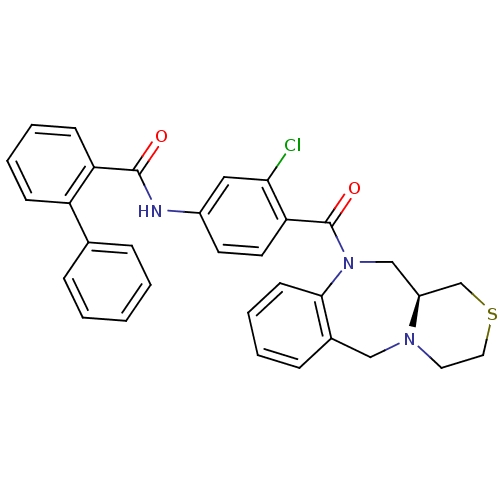
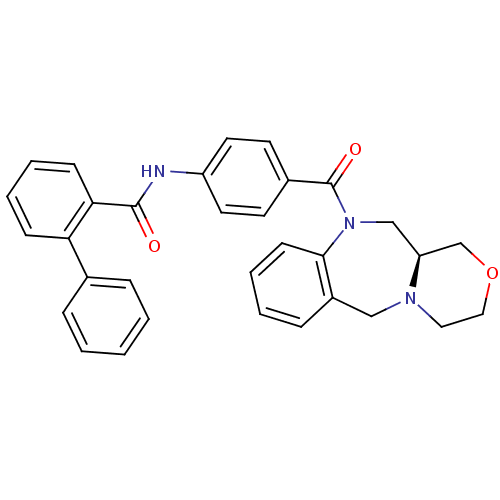
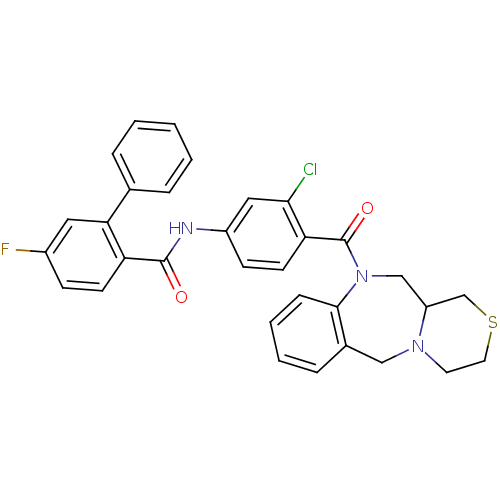
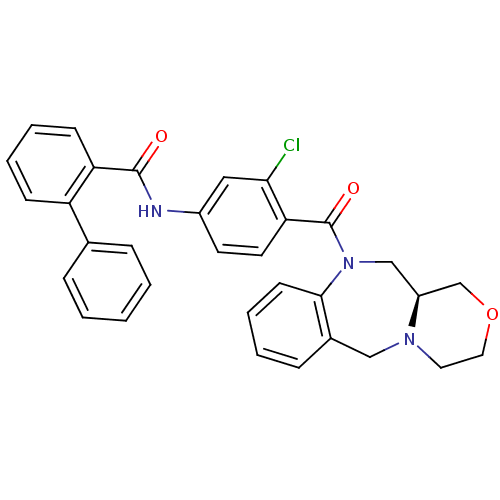
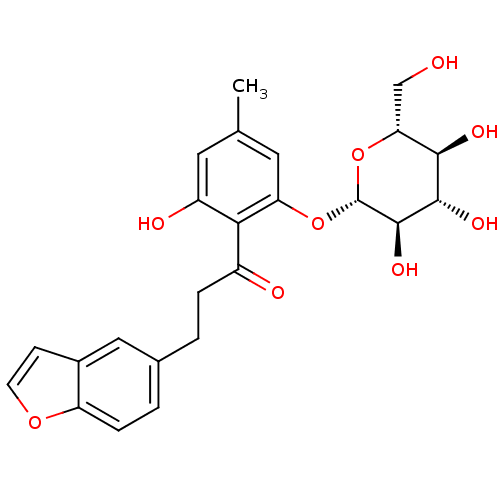
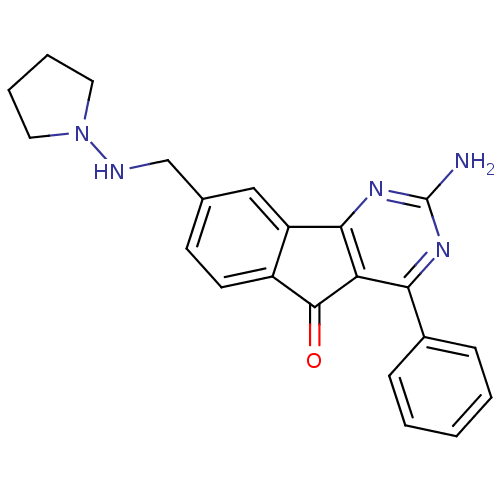
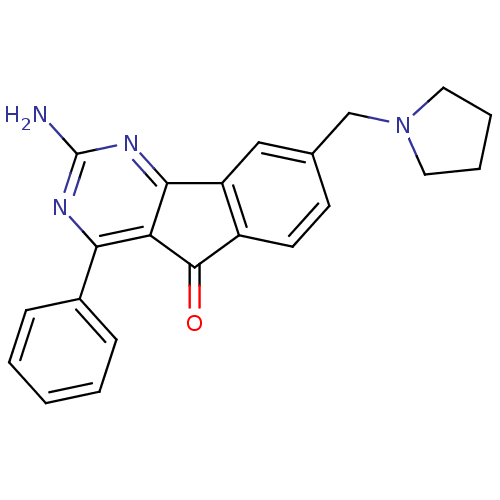
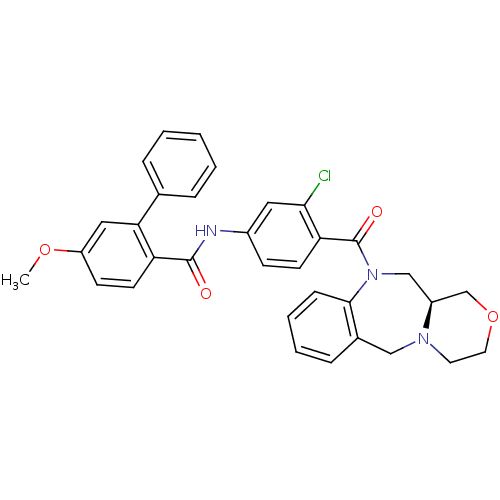
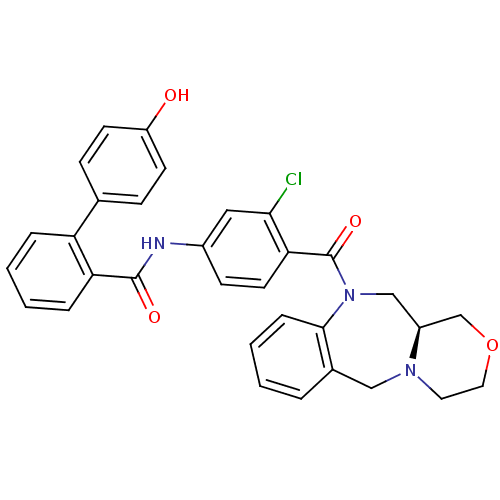
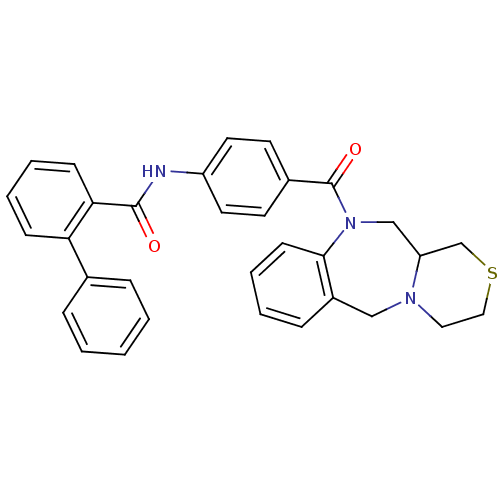
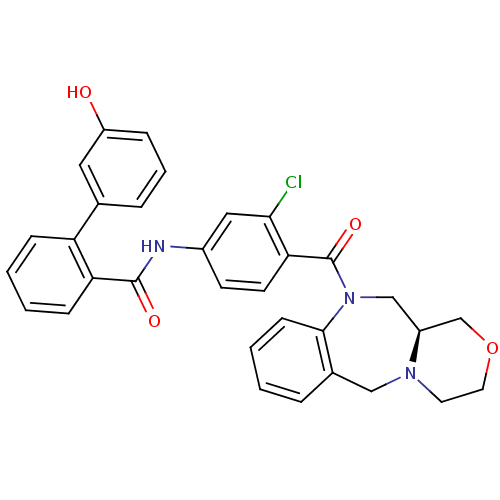
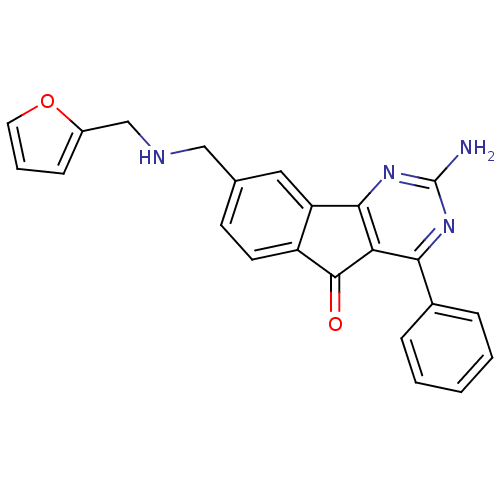
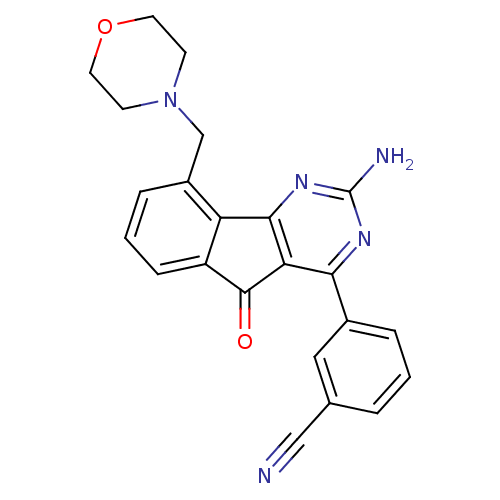
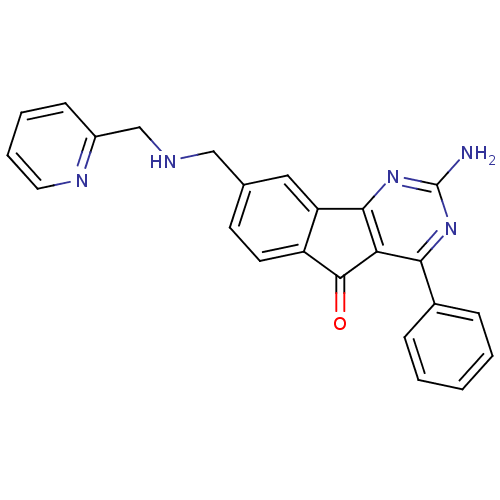
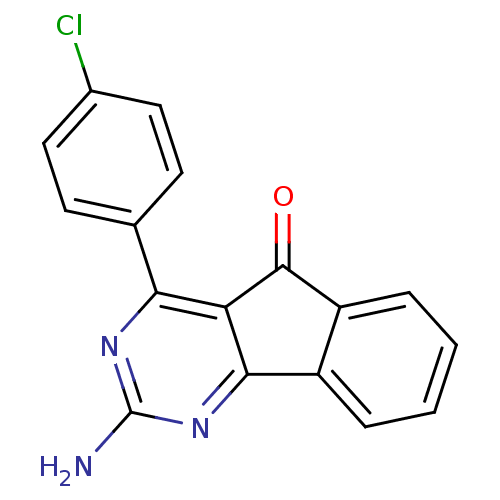
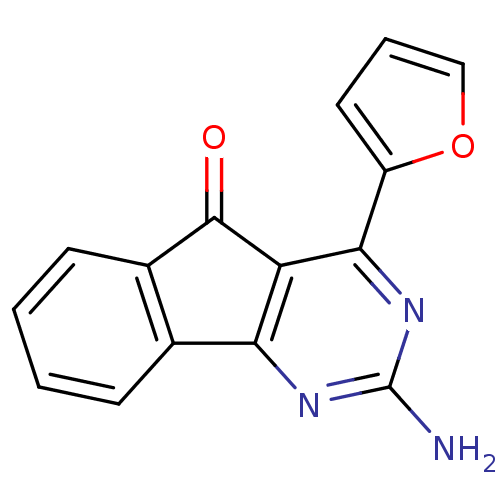
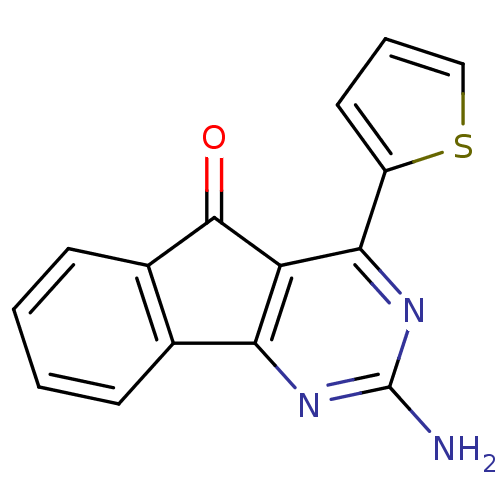
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Antagonist activity at adenosine A2A receptorMore data for this Ligand-Target Pair
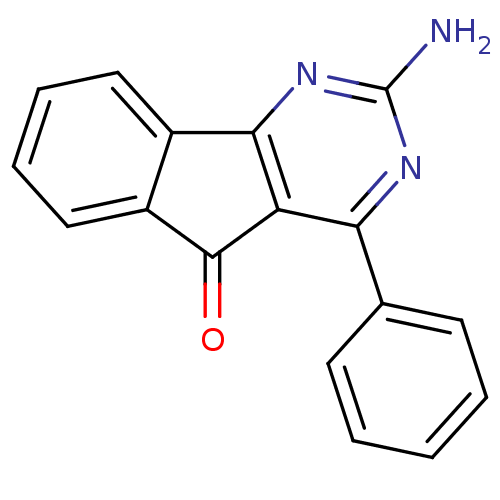
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Antagonist activity at adenosine A2A receptorMore data for this Ligand-Target Pair
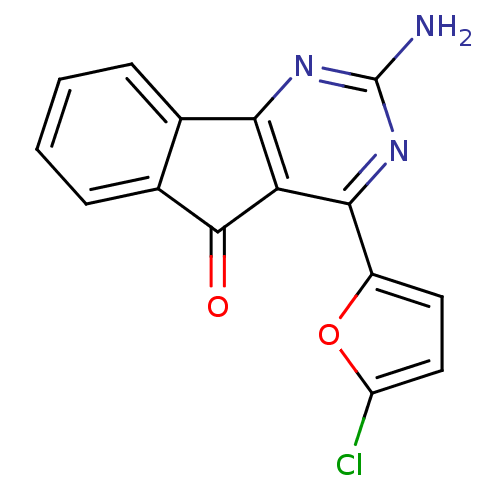
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Antagonist activity at adenosine A2A receptorMore data for this Ligand-Target Pair
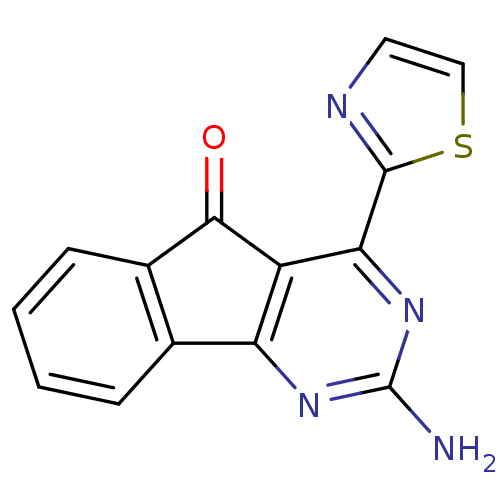
TargetAdenosine receptor A1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Antagonist activity at adenosine A1 receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Antagonist activity at adenosine A1 receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Antagonist activity at adenosine A2A receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Antagonist activity at adenosine A2A receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Antagonist activity at adenosine A2A receptorMore data for this Ligand-Target Pair
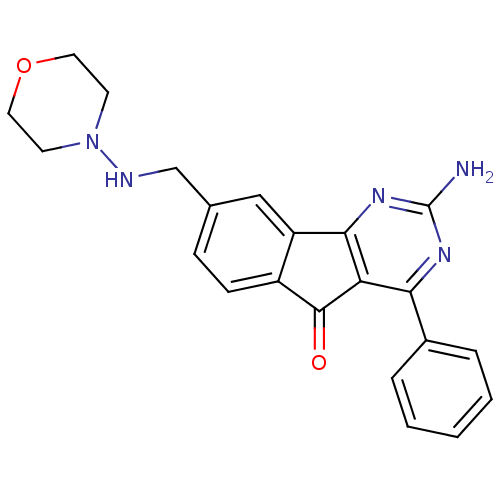
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic...More data for this Ligand-Target Pair
TargetAdenosine receptor A1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Antagonist activity at adenosine A1 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.60nMAssay Description:Antagonist activity at adenosine A2A receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Antagonist activity at adenosine A1 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.90nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Antagonist activity at adenosine A1 receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Antagonist activity at adenosine A2A receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.80nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V1a receptorMore data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic...More data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Antagonist activity at adenosine A2A receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:Antagonist activity at adenosine A1 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 3.30nMAssay Description:Antagonist activity at adenosine A2A receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 3.60nMAssay Description:Antagonist activity at adenosine A2A receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 3.80nMAssay Description:Antagonist activity at adenosine A2A receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetSodium/glucose cotransporter 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:In vitro inhibition of Na-dependent [14C]-AMG uptake in CHO-K1 cells expressing human sodium glucose co-transporter 2More data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 4.10nMAssay Description:Antagonist activity at adenosine A2A receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 4.10nMAssay Description:Antagonist activity at adenosine A2A receptorChecked by AuthorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4.20nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4.20nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 4.60nMAssay Description:Antagonist activity at adenosine A2A receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 5.30nMAssay Description:Antagonist activity at adenosine A2A receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 5.40nMAssay Description:Antagonist activity at adenosine A2A receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 5.40nMAssay Description:Antagonist activity at adenosine A2A receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 5.5nMAssay Description:Antagonist activity at adenosine A2A receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 5.90nMAssay Description:Antagonist activity at adenosine A2A receptorMore data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)