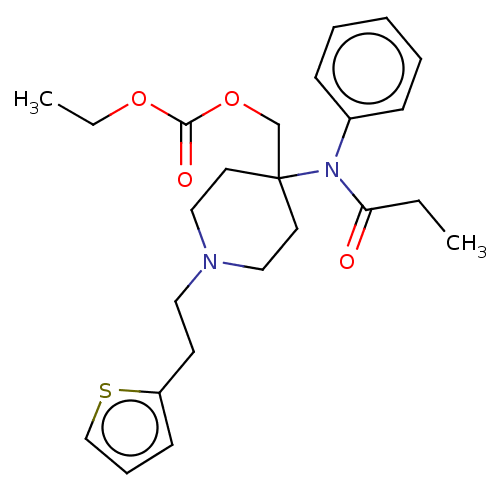
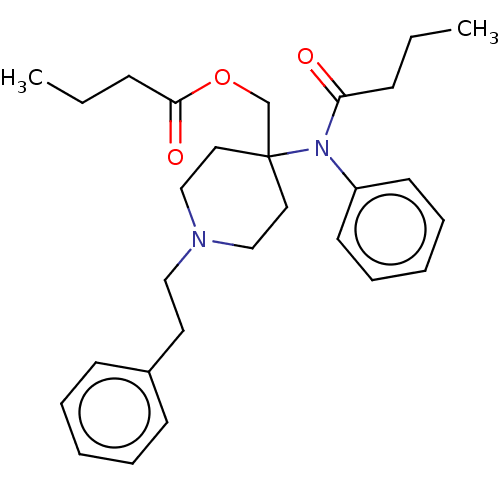
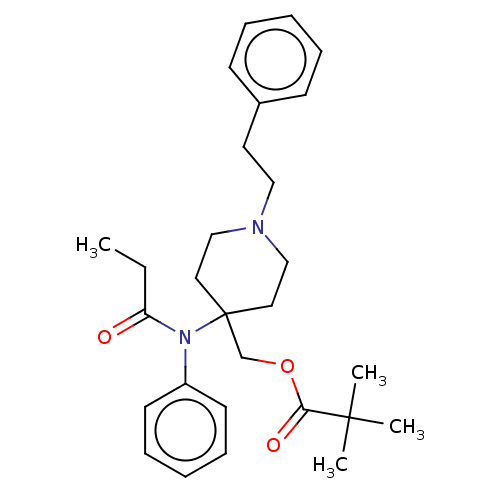
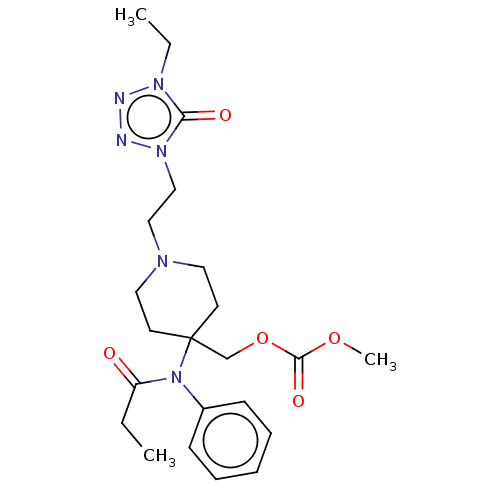
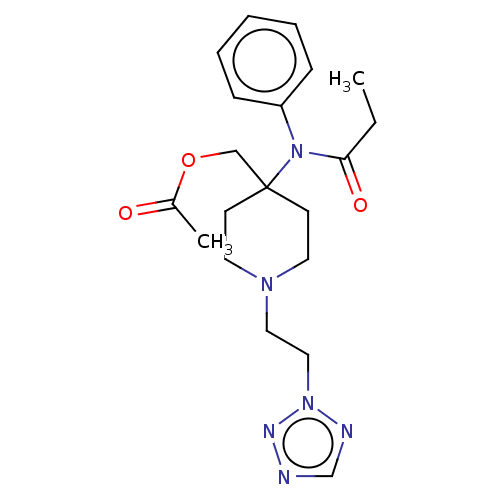
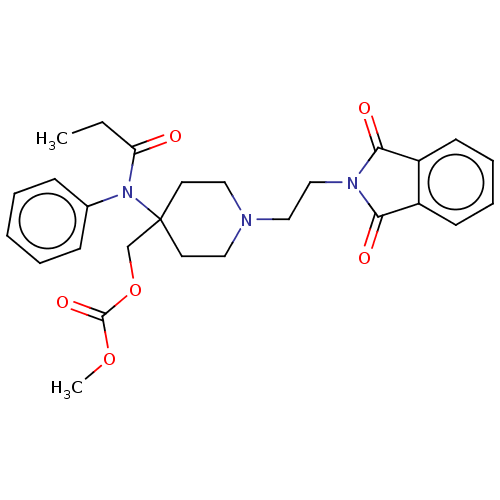
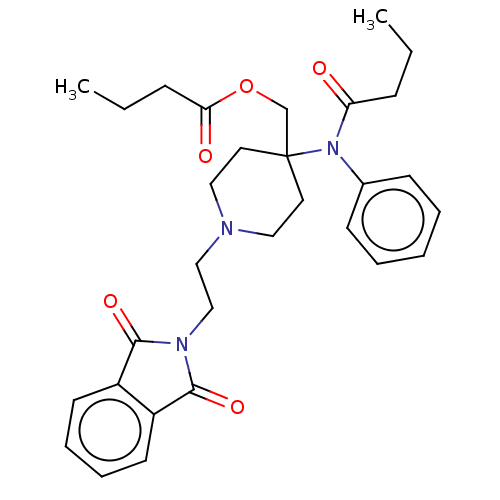
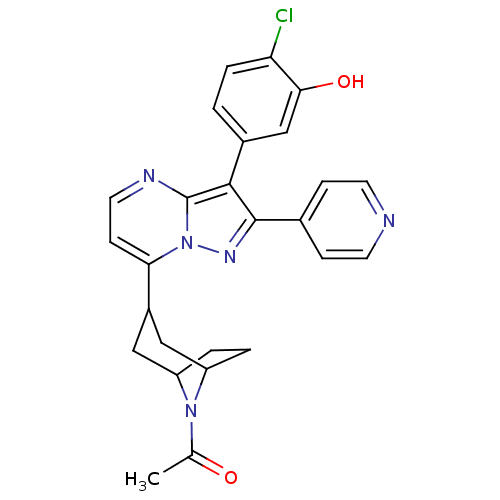
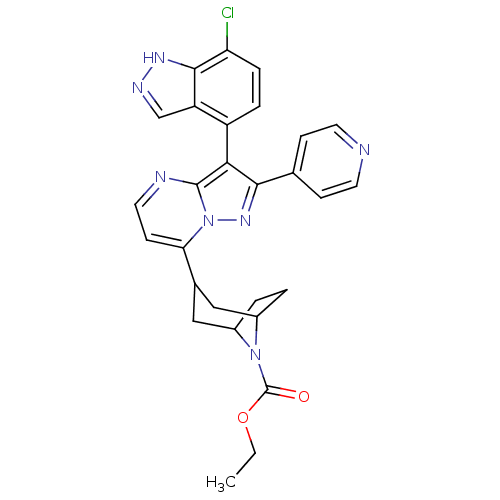
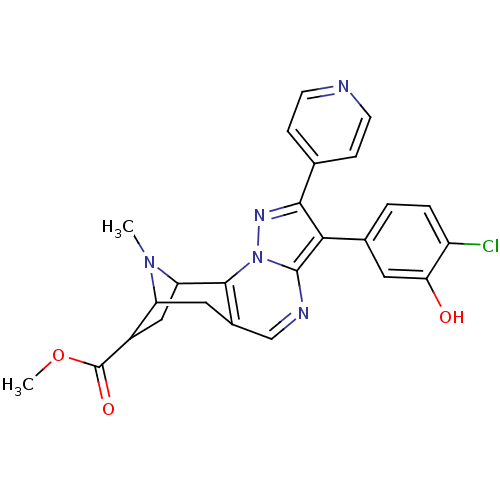
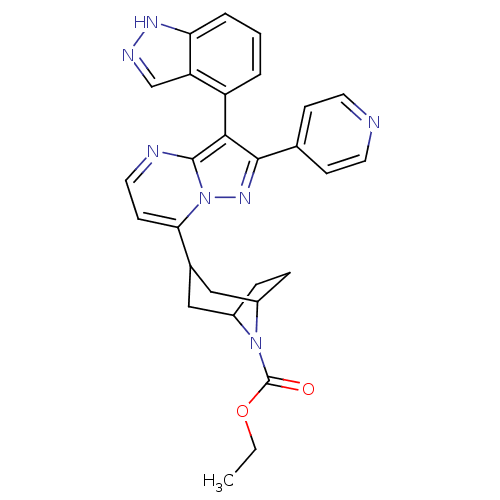
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.0200nMAssay Description:In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
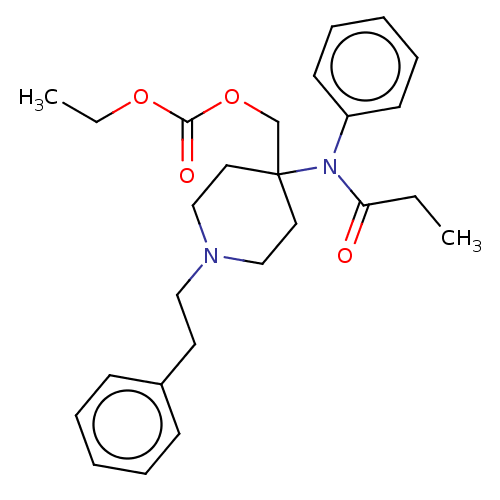
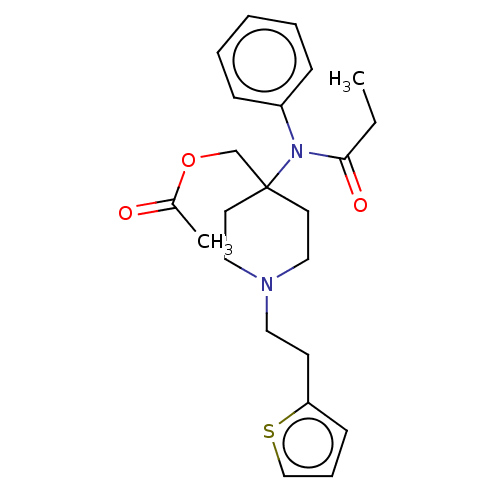
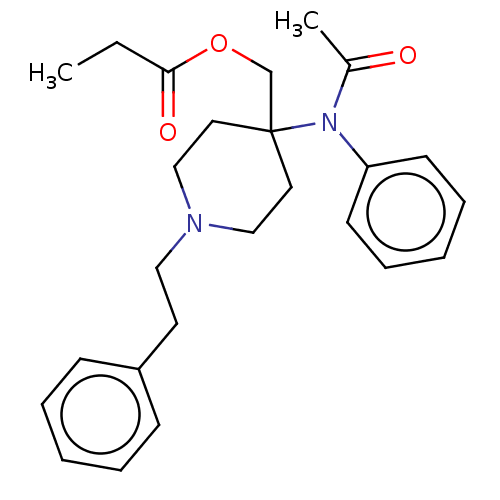
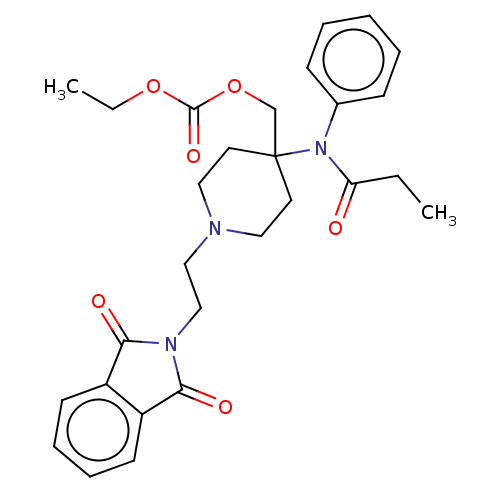
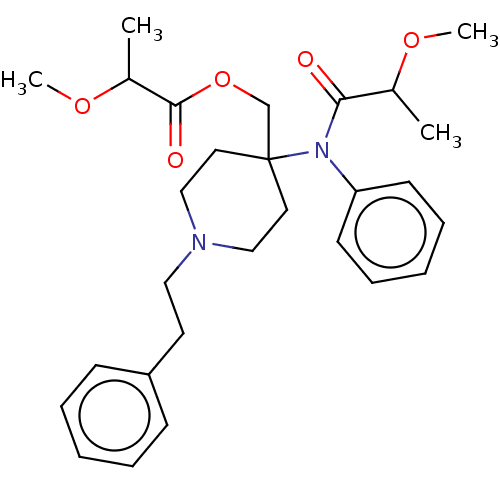
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 2.20nMAssay Description:In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
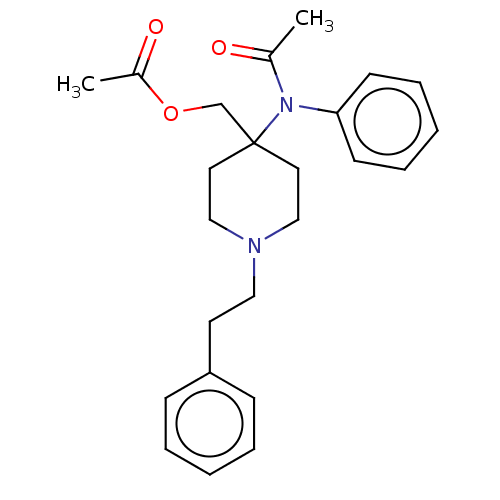
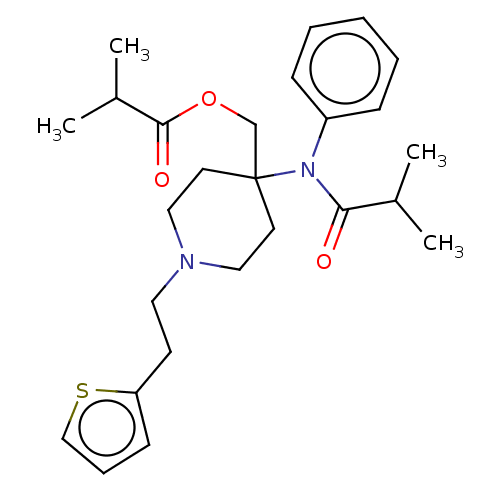
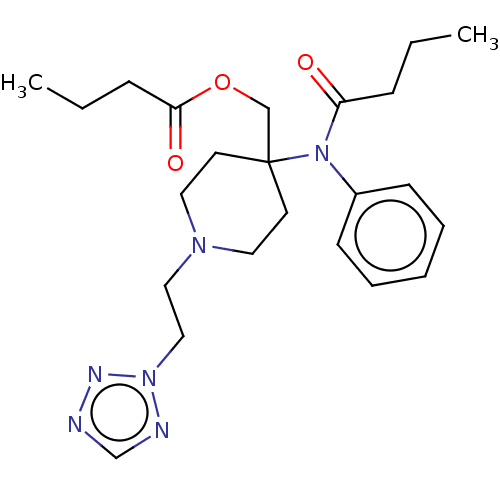
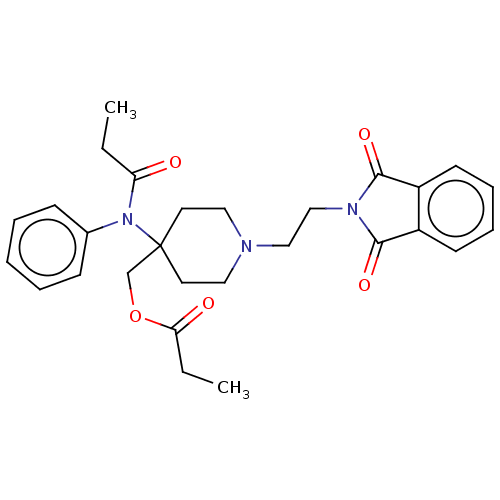
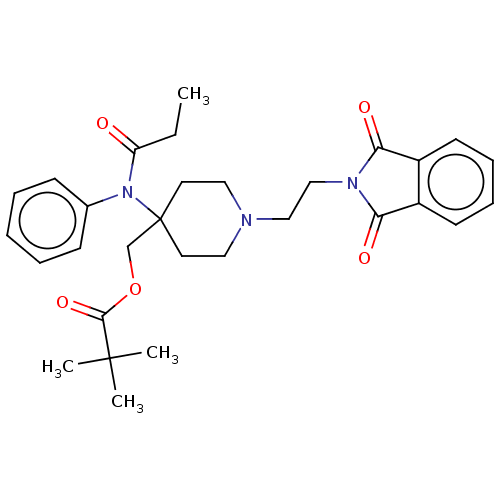
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 3.5nMAssay Description:Antimuscarinic activity on the acetylcholine-induced inhibition of contraction of guinea pig ileum which has Muscarinic acetylcholine receptor M2 sub...More data for this Ligand-Target Pair
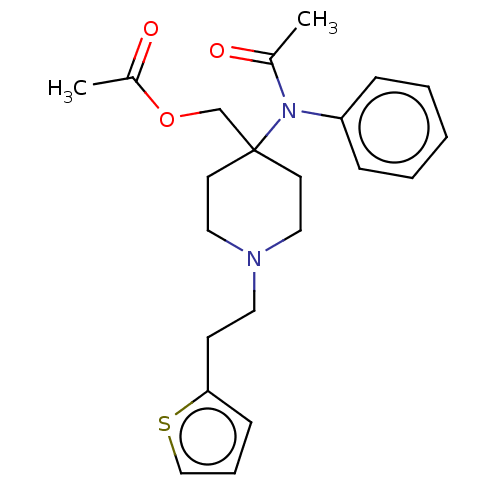
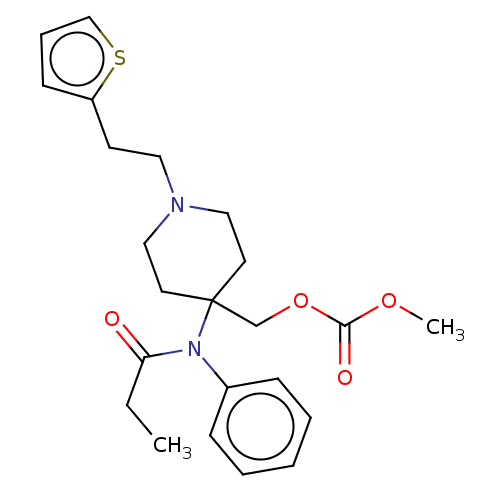
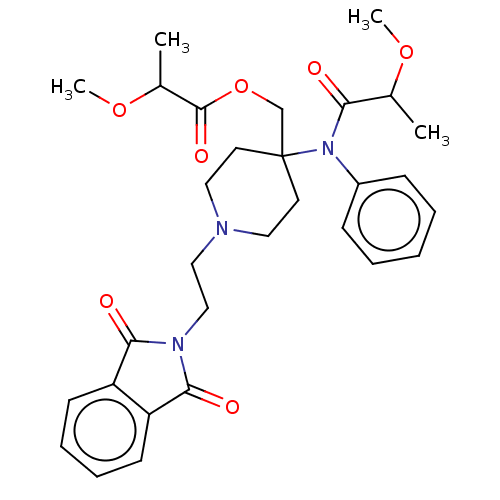
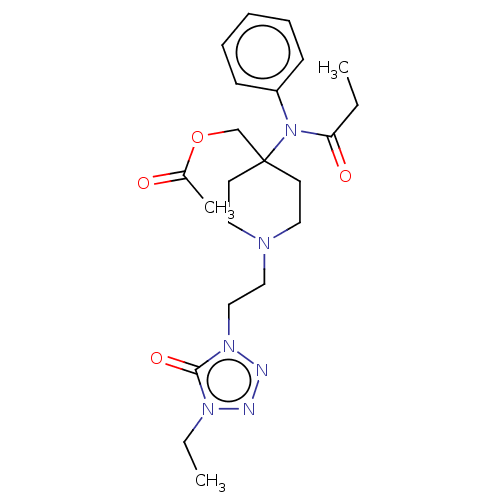
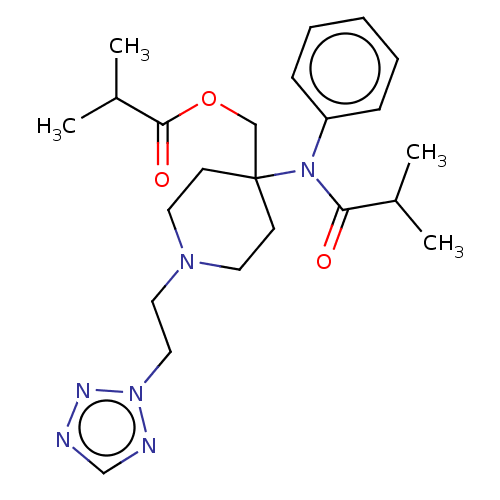
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 7.70nMAssay Description:In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 8.20nMAssay Description:Antimuscarinic activity on the acetylcholine-induced inhibition of contraction of guinea pig ileum which has Muscarinic acetylcholine receptor M2 sub...More data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 8.20nMAssay Description:In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 9.10nMAssay Description:In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 24nMAssay Description:Antimuscarinic activity on the acetylcholine-induced inhibition of contraction of guinea pig ileum which has M2 muscarinic receptor subtype.More data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 26nMAssay Description:In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 27nMAssay Description:In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 30nMAssay Description:In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 30nMAssay Description:Antimuscarinic activity on the acetylcholine-induced inhibition of contraction of guinea pig ileum which has M2 muscarinic receptor subtype.More data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 41nMAssay Description:In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 49nMAssay Description:In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 56nMAssay Description:In vitro affinity to displace [3H]naloxone from opioid receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:In vitro affinity to displace [3H]naloxone from opioid receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:In vitro affinity to displace [3H]naloxone from opioid receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:In vitro affinity to displace [3H]naloxone from opioid receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:In vitro affinity to displace [3H]naloxone from opioid receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:In vitro affinity to displace [3H]naloxone from opioid receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:In vitro affinity to displace [3H]naloxone from opioid receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:In vitro affinity to displace [3H]naloxone from opioid receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:In vitro affinity to displace [3H]naloxone from opioid receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:In vitro affinity to displace [3H]naloxone from opioid receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:In vitro affinity to displace [3H]naloxone from opioid receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:In vitro affinity to displace [3H]naloxone from opioid receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:Antimuscarinic activity on the acetylcholine-induced inhibition of contraction of guinea pig ileum which has Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:Antimuscarinic activity on the acetylcholine-induced inhibition of contraction of guinea pig ileum which has M2 muscarinic receptor subtype.More data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:Antimuscarinic activity on the acetylcholine-induced inhibition of contraction of guinea pig ileum which has M2 muscarinic receptor subtype.More data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:Antimuscarinic activity on the acetylcholine-induced inhibition of contraction of guinea pig ileum which has M2 muscarinic receptor subtype.More data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:In vitro affinity to displace [3H]naloxone from opioid receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rattus norvegicus (rat))
Anaquest Pharmaceuticals
Curated by ChEMBL
Anaquest Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >100nMAssay Description:In vitro affinity to displace [3H]naloxone from opioid receptor in freshly prepared rat brain homogenatesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of B-RafMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of B-RafMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of B-RafMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of B-RafMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibition of B-RafMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inhibition of B-RafMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibition of B-RafMore data for this Ligand-Target Pair

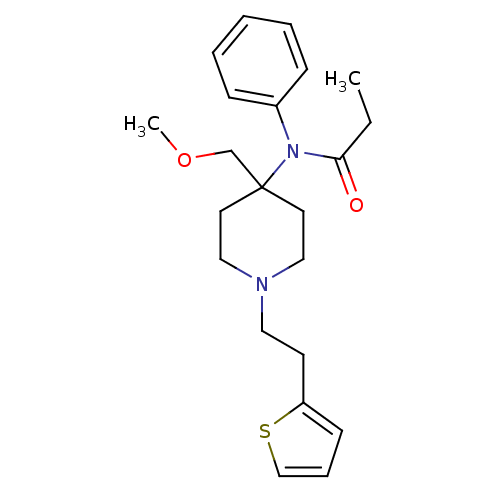
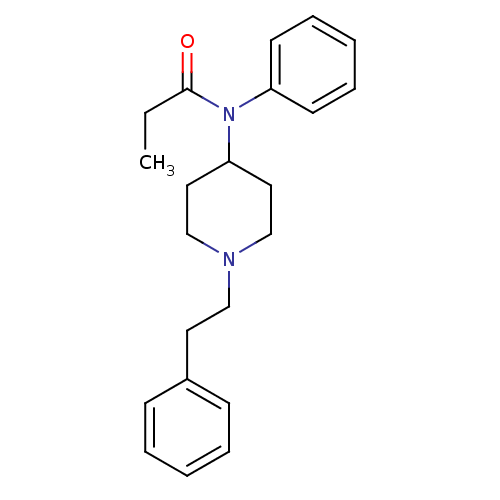
 3D Structure (crystal)
3D Structure (crystal)