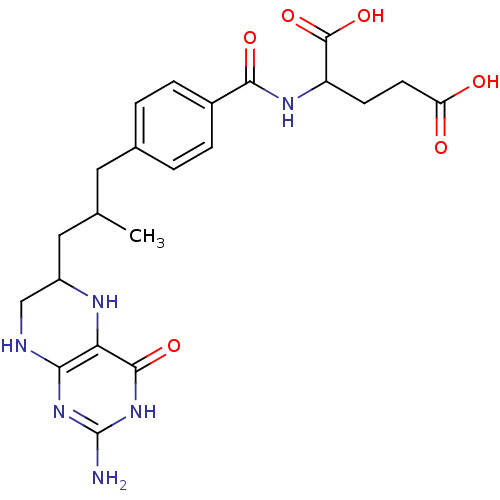
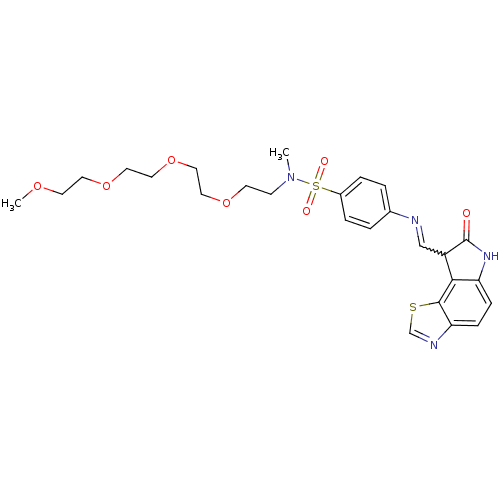
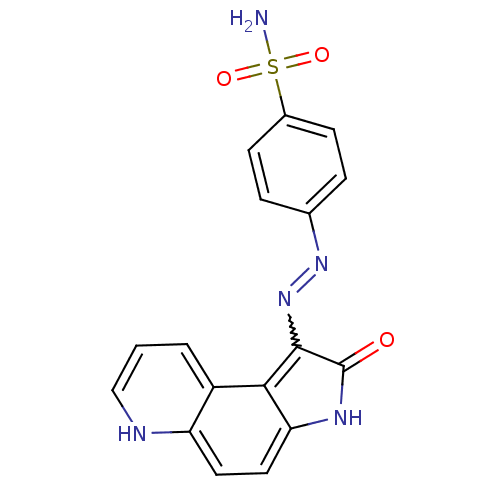
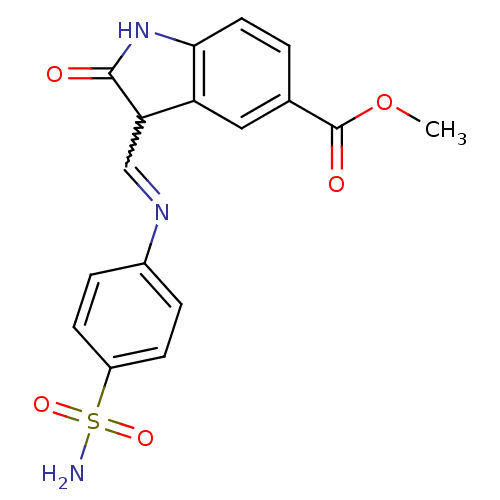
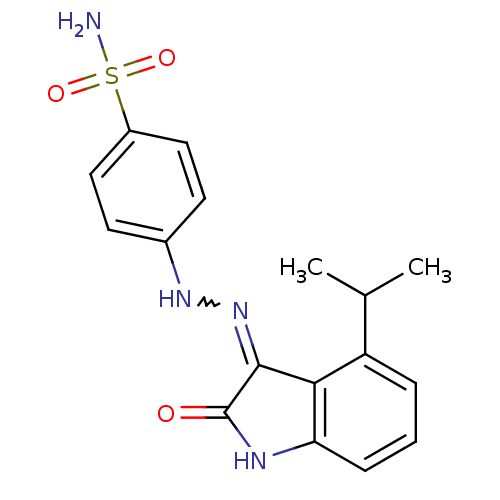
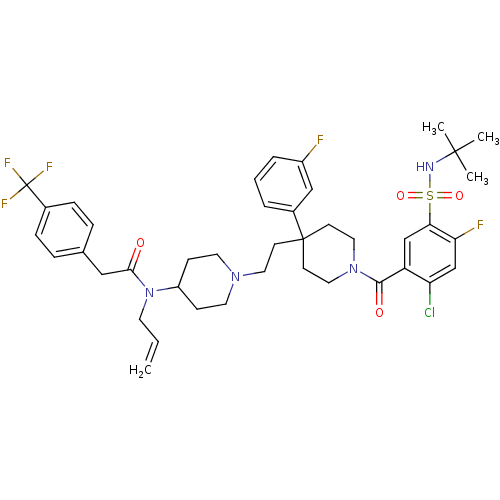
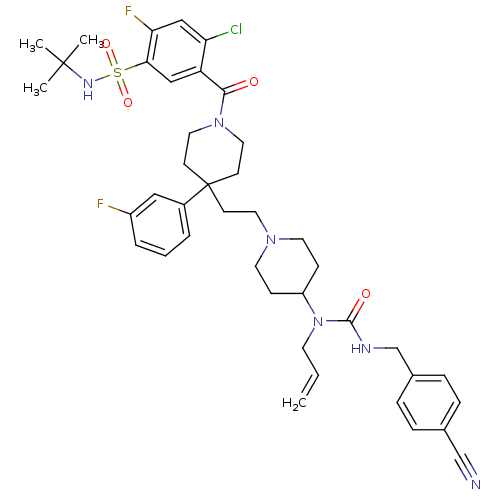
TargetTrifunctional purine biosynthetic protein adenosine-3(Homo sapiens (Human))
University Of South Alabama
Curated by ChEMBL
University Of South Alabama
Curated by ChEMBL
Affinity DataIC50: 0nMAssay Description:Inhibition of the GAR transformylase in lactobacillus caseiMore data for this Ligand-Target Pair
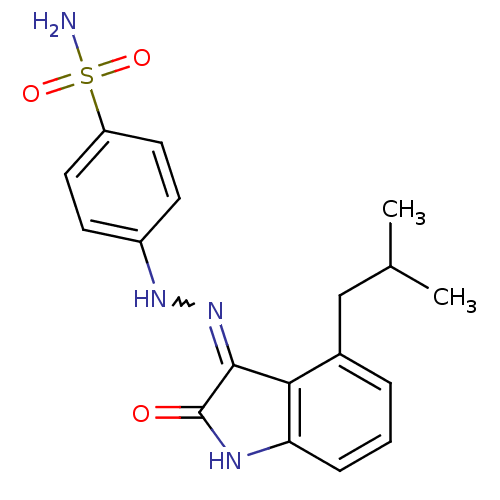
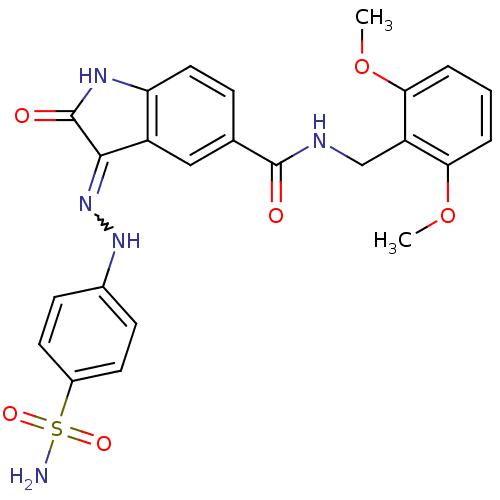
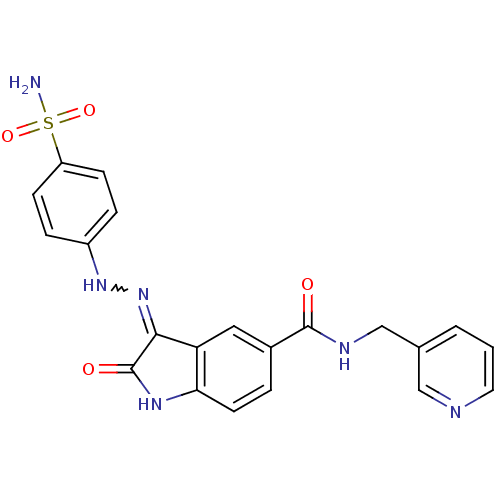
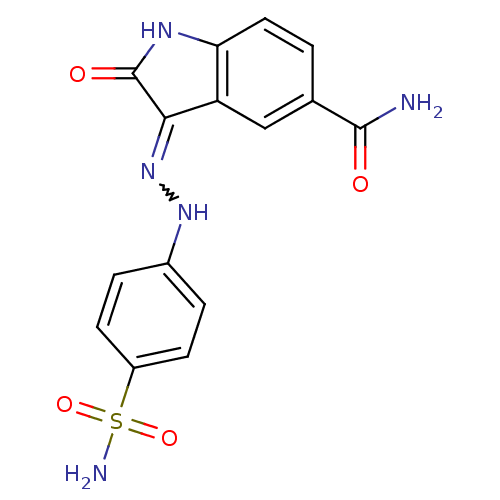
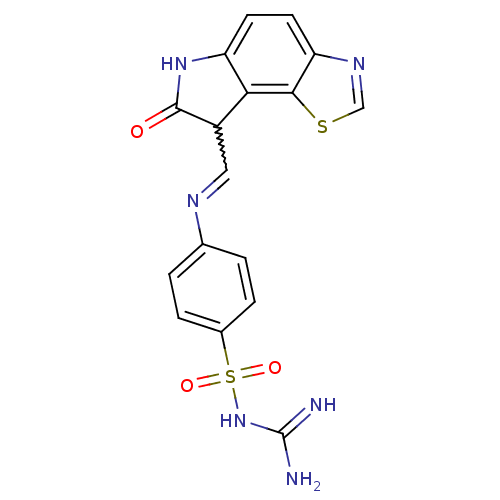
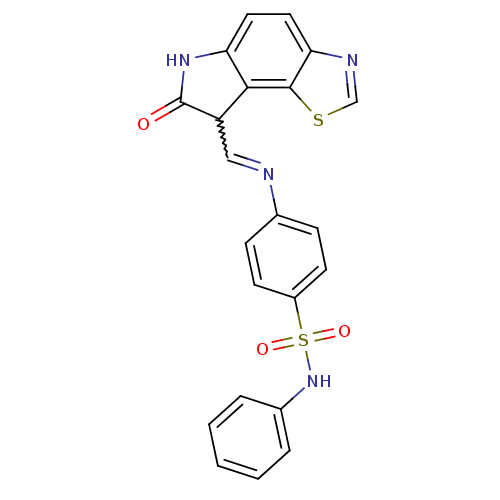
TargetTrifunctional purine biosynthetic protein adenosine-3(Homo sapiens (Human))
University Of South Alabama
Curated by ChEMBL
University Of South Alabama
Curated by ChEMBL
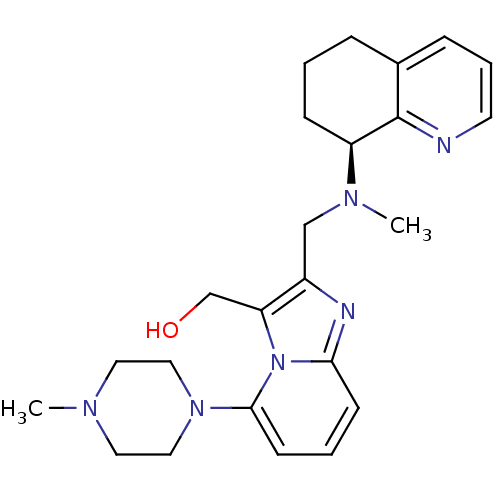
Affinity DataIC50: 0.00100nMAssay Description:Inhibition of the GAR transformylase in lactobacillus caseiMore data for this Ligand-Target Pair
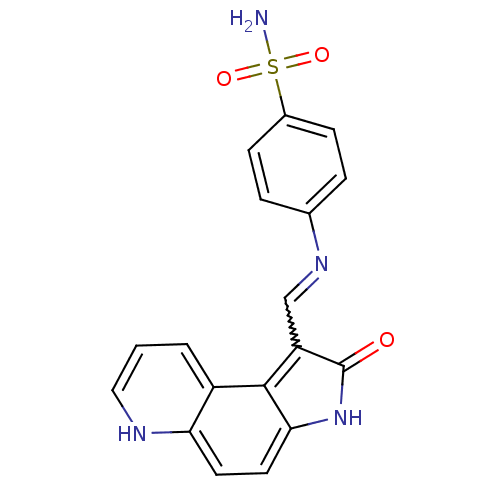
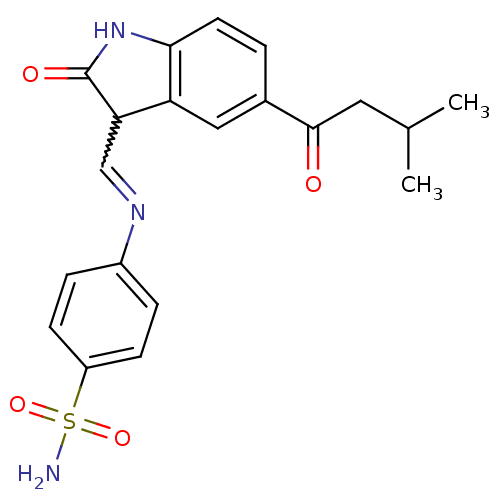
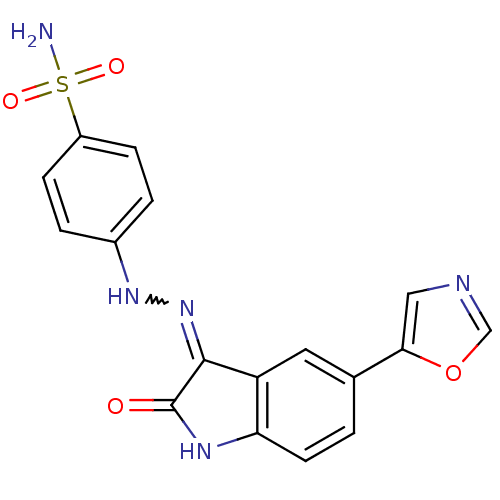
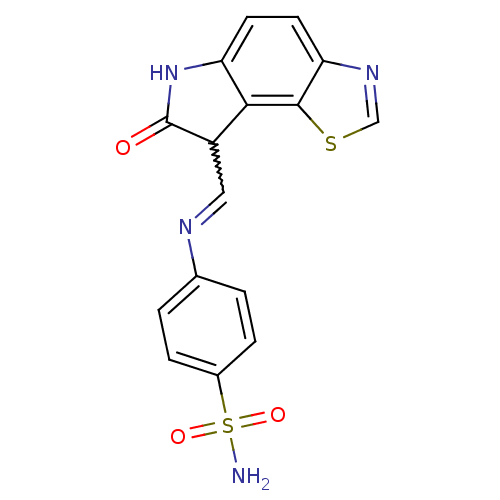
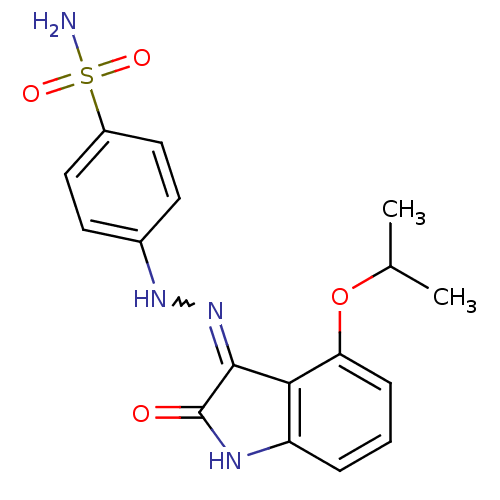
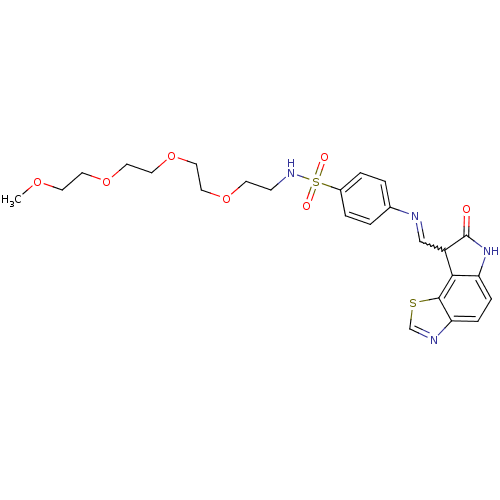
TargetTrifunctional purine biosynthetic protein adenosine-3(Homo sapiens (Human))
University Of South Alabama
Curated by ChEMBL
University Of South Alabama
Curated by ChEMBL
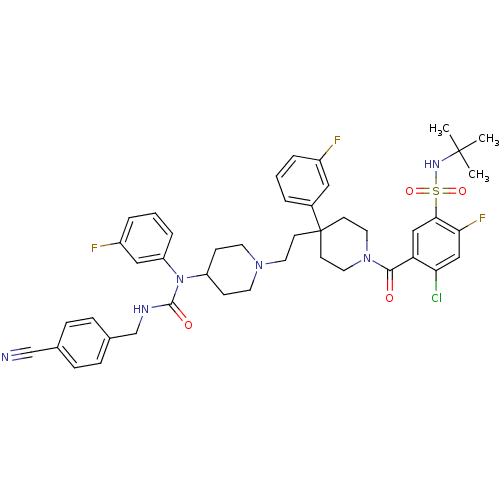
Affinity DataIC50: 0.00100nMAssay Description:Inhibition of the GAR transformylase in lactobacillus caseiMore data for this Ligand-Target Pair
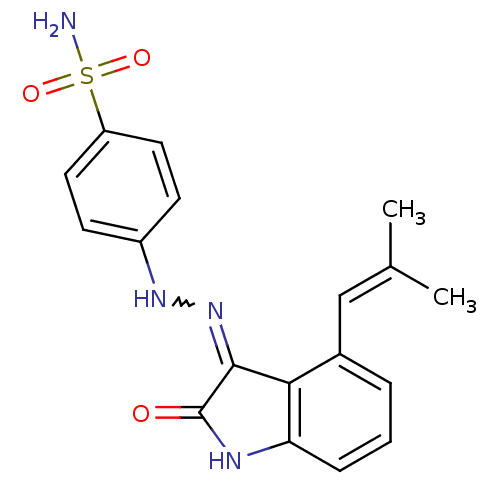
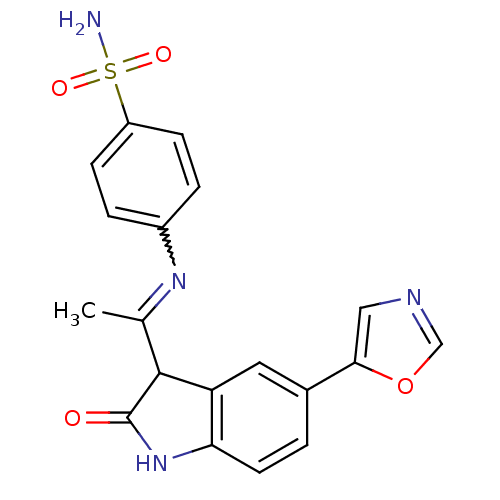
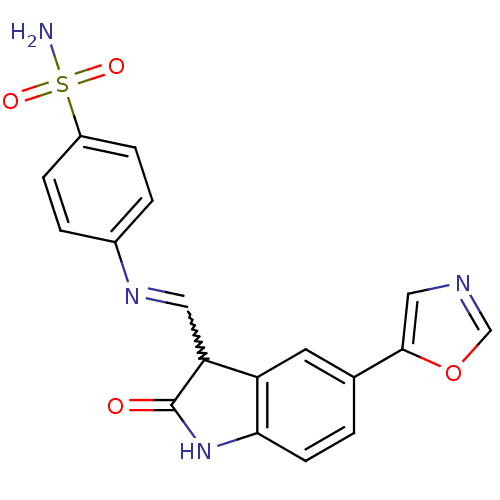
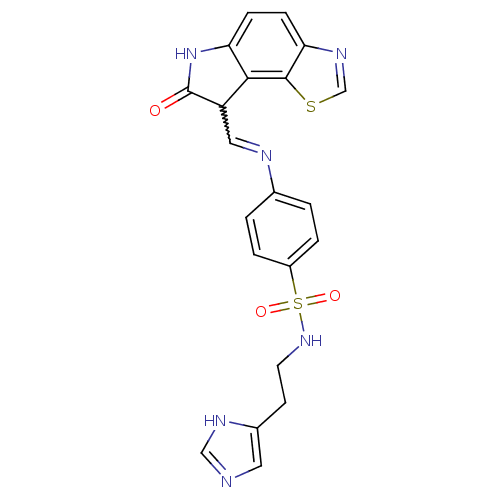
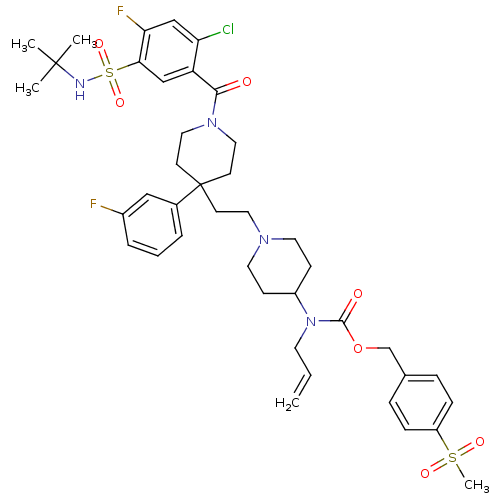
TargetTrifunctional purine biosynthetic protein adenosine-3(Homo sapiens (Human))
University Of South Alabama
Curated by ChEMBL
University Of South Alabama
Curated by ChEMBL
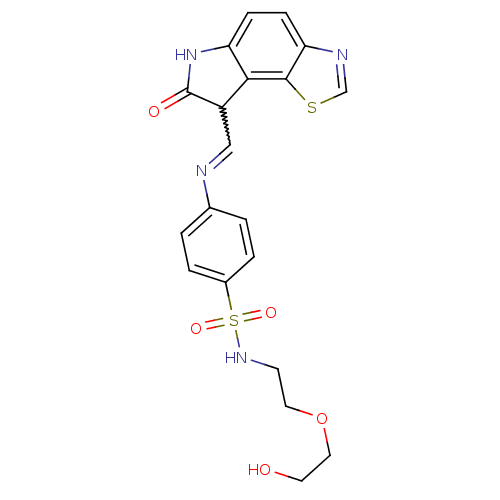
Affinity DataIC50: 0.00100nMAssay Description:Inhibition of the GAR transformylase in lactobacillus caseiMore data for this Ligand-Target Pair
TargetTrifunctional purine biosynthetic protein adenosine-3(Homo sapiens (Human))
University Of South Alabama
Curated by ChEMBL
University Of South Alabama
Curated by ChEMBL
Affinity DataIC50: 0.0140nMAssay Description:Inhibition of the GAR transformylase in MOLT-4 human leukemia cellsMore data for this Ligand-Target Pair
TargetTrifunctional purine biosynthetic protein adenosine-3(Mus musculus)
University Of South Alabama
Curated by ChEMBL
University Of South Alabama
Curated by ChEMBL
Affinity DataIC50: 0.0180nMAssay Description:Inhibition of the GAR transformylase in L1210More data for this Ligand-Target Pair
TargetTrifunctional purine biosynthetic protein adenosine-3(Mus musculus)
University Of South Alabama
Curated by ChEMBL
University Of South Alabama
Curated by ChEMBL
Affinity DataIC50: >0.0200nMAssay Description:Inhibition of the GAR transformylase in L1210More data for this Ligand-Target Pair
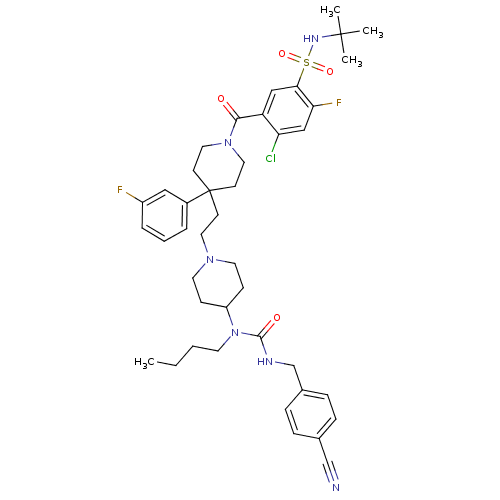
Affinity DataIC50: 0.340nMAssay Description:Inhibition of CXCR4-mediated chemotaxis in SDF1-stimulated human U937 cells treated 15 mins before SDF1 challenge measured after 2 hrs by luminescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infectionMore data for this Ligand-Target Pair
Affinity DataIC50: 0.540nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 group M subtyp...)
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.560nMAssay Description:Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.870nMAssay Description:Antagonist activity at human CXCR4 expressed in HEK293 cells assessed as inhibition of SDF1-induced response treated 30 mins before agonist challenge...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 1.07nMAssay Description:Antagonist activity at human CXCR4 expressed in HEK293 cells assessed as inhibition of SDF1-induced response treated before agonist challenge measure...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infectionMore data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 group M subtyp...)
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 1.71nMAssay Description:Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assay in presence of 45 mg/ml human ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 group M subtyp...)
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 1.97nMAssay Description:Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assay in presence of alpha-acid glyc...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 group M subtyp...)
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 1.99nMAssay Description:Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assay in presence of 1 mg/ml alpha-a...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Antagonist activity at mouse CXCR4 expressed in human U2OS cells assessed as inhibition of SDF1-induced increase in intracellular calcium level by FL...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 2.41nMAssay Description:Antagonist activity at human CXCR4 expressed in HEK293 cells assessed as inhibition of SDF1-induced increase in intracellular calcium level treated 1...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infectionMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 3.30nMAssay Description:Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infectionMore data for this Ligand-Target Pair
Affinity DataIC50: 3.30nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 3.36nMAssay Description:Antagonist activity at human CXCR4 expressed in HEK293 cells assessed as inhibition of SDF1-induced response treated before agonist challenge measure...More data for this Ligand-Target Pair
Affinity DataIC50: 3.40nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infectionMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90nMAssay Description:Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infectionMore data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMAssay Description:Antagonist activity at rat CXCR4 expressed in human U2OS cells assessed as inhibition of SDF1-induced increase in intracellular calcium level by FLIP...More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infectionMore data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infectionMore data for this Ligand-Target Pair