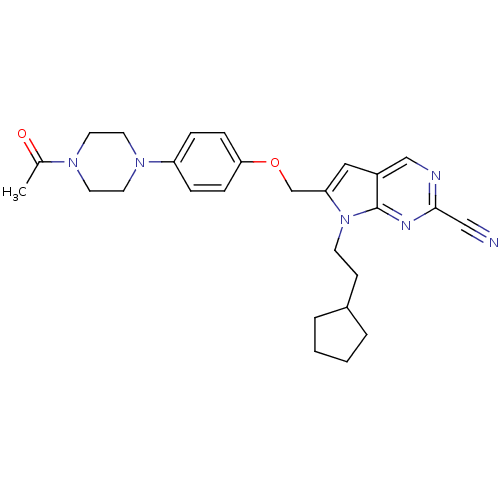
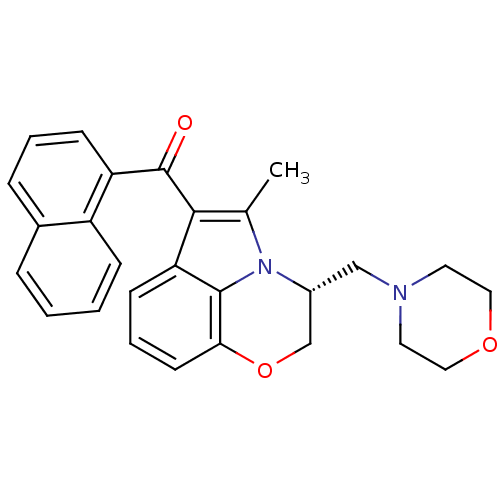
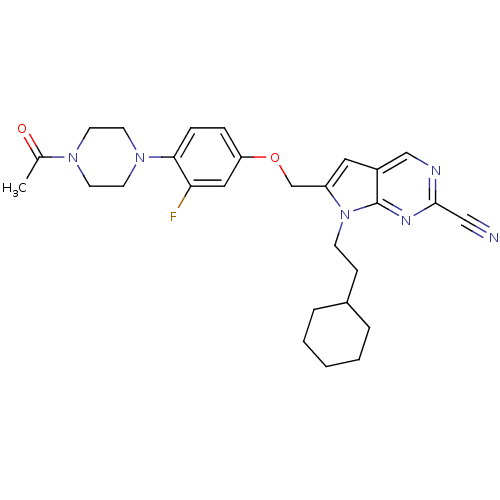
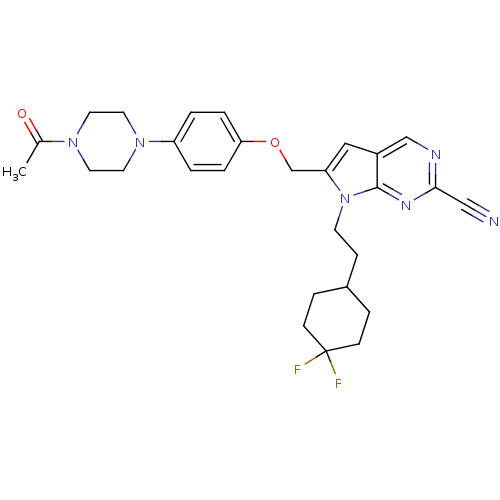
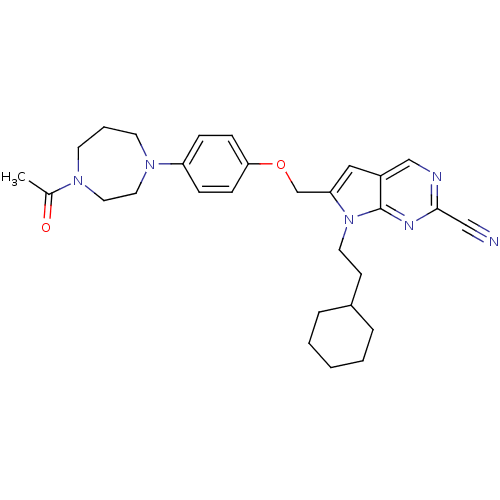
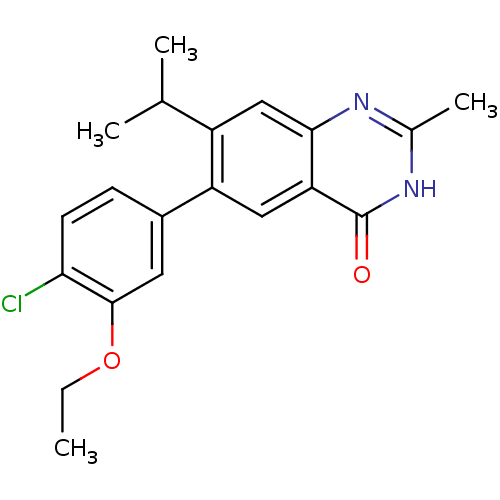
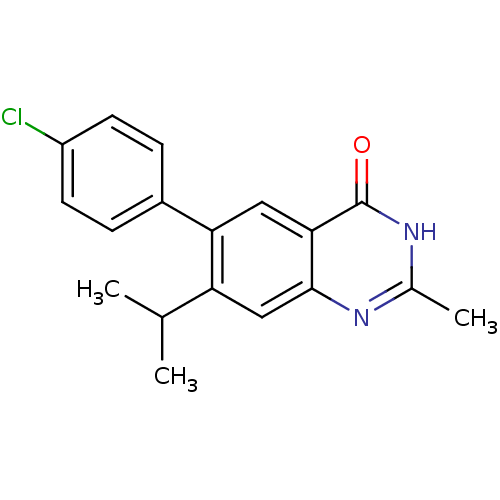
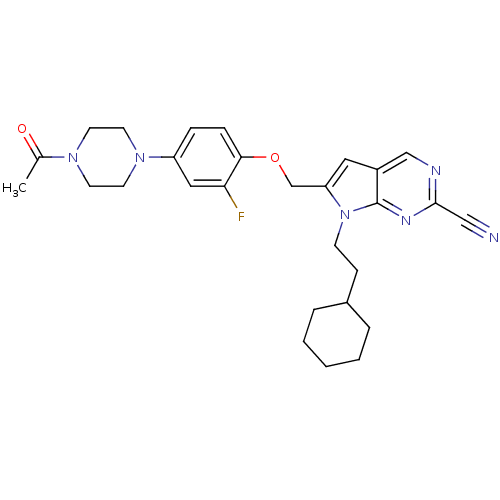
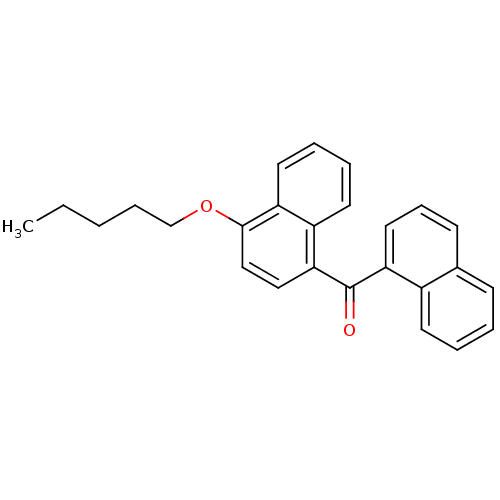
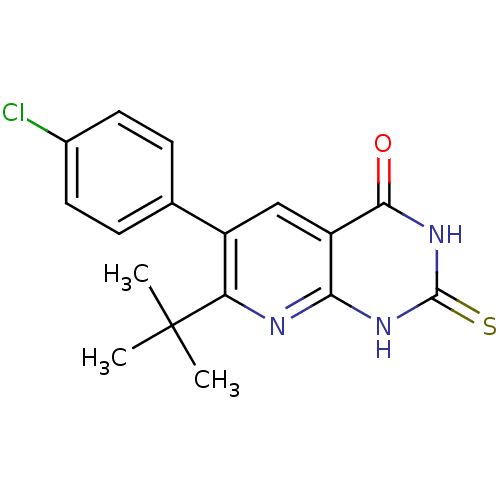
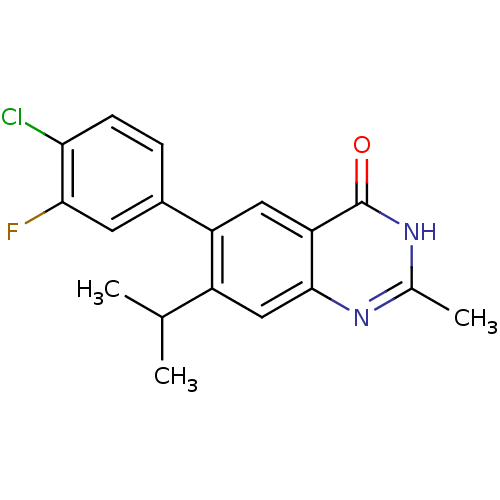
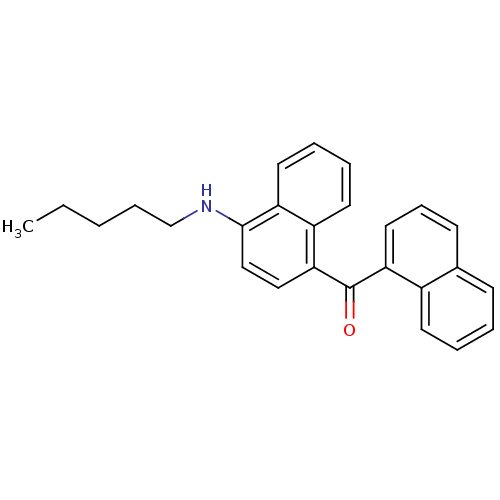
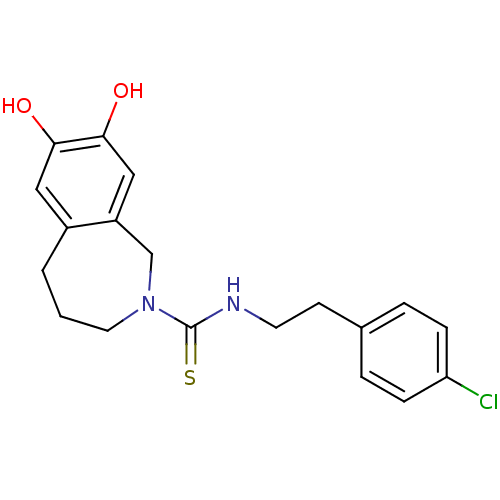
TargetCannabinoid receptor 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.770nMAssay Description:Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 8.90nMAssay Description:Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of calcium influx evoked by capsaicin in human TRPV1 expressing cells by fluorescence assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of calcium influx evoked by capsaicin in human TRPV1 expressing cells by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of calcium influx evoked by capsaicin in human TRPV1 expressing cells by fluorescence assayMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of low pH activation of human TRPV1More data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Rattus norvegicus (rat))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Inhibition of rat CB1 receptorMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:Inhibition of calcium influx evoked by capsaicin in human TRPV1 expressing cells by fluorescence assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Inhibition of low pH activation of rat TRPV1More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 31nMAssay Description:Inhibition of calcium influx evoked by capsaicin in human TRPV1 expressing cells by fluorescence assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 37nMAssay Description:Inhibition of calcium influx evoked by capsaicin in human TRPV1 expressing cells by fluorescence assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of calcium influx evoked by capsaicin in human TRPV1 expressing cells by fluorescence assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 42nMAssay Description:Inhibition of calcium influx evoked by capsaicin in human TRPV1 expressing cells by fluorescence assayMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 48nMAssay Description:Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 49nMAssay Description:Inhibition of low pH activation of human TRPV1More data for this Ligand-Target Pair
Affinity DataIC50: 50nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of calcium influx evoked by capsaicin in human TRPV1 expressing cells by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 79nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 82nMAssay Description:Inhibition of calcium influx evoked by capsaicin in human TRPV1 expressing cells by fluorescence assayMore data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 85nMAssay Description:Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 90nMAssay Description:Inhibition of calcium influx evoked by capsaicin in rat TRPV1 expressing cells by fluorescence assayMore data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 90nMAssay Description:Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cellsMore data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 98nMAssay Description:Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of calcium influx evoked by capsaicin in human TRPV1 expressing cells by fluorescence assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of calcium influx evoked by capsaicin in human TRPV1 expressing cells by fluorescence assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 105nMAssay Description:Inhibition of low pH activation of rat TRPV1More data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 133nMAssay Description:Inhibition of calcium influx evoked by capsaicin in human TRPV1 expressing cells by fluorescence assayMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 144nMAssay Description:Inhibition of calcium influx evoked by capsaicin in human TRPV1 expressing cells by fluorescence assayMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 160nMAssay Description:Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 201nMAssay Description:Inhibition of calcium influx evoked by capsaicin in human TRPV1 expressing cells by fluorescence assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 216nMAssay Description:Inhibition of calcium influx evoked by capsaicin in human TRPV1 expressing cells by fluorescence assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 286nMAssay Description:Inhibition of calcium influx evoked by capsaicin in human TRPV1 expressing cells by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 290nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 334nMAssay Description:Inhibition of calcium influx evoked by capsaicin in human TRPV1 expressing cells by fluorescence assayMore data for this Ligand-Target Pair

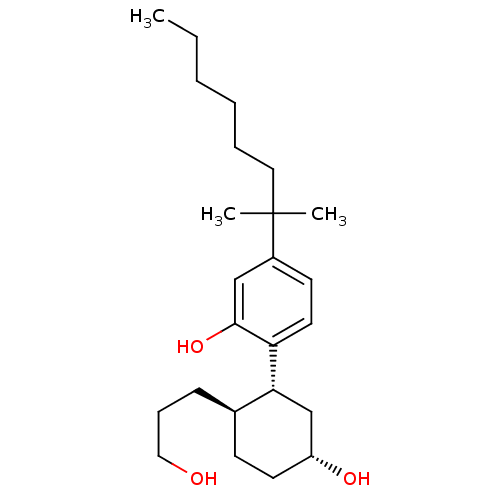
 3D Structure (crystal)
3D Structure (crystal)