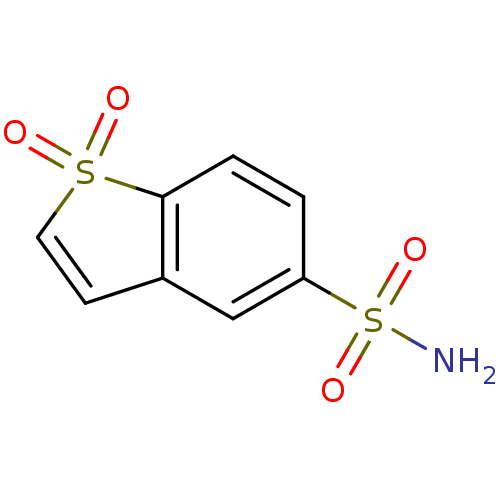
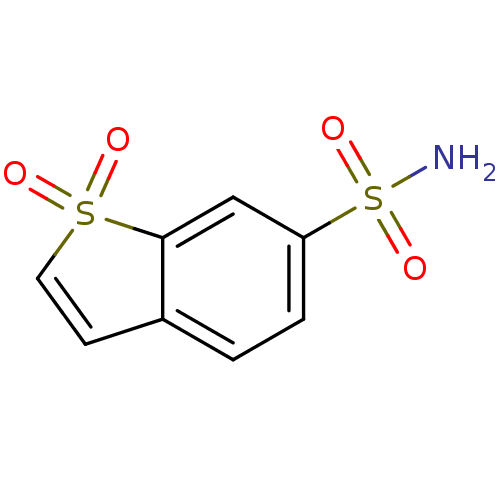
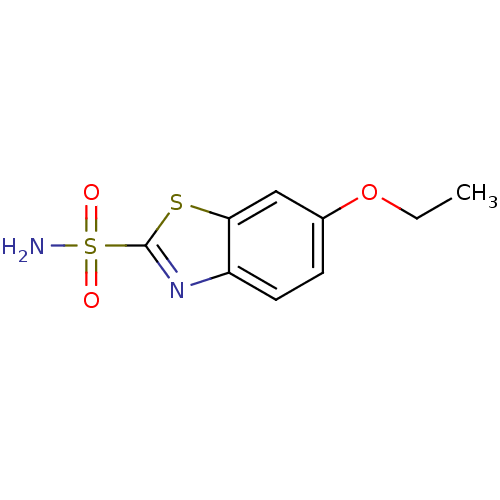
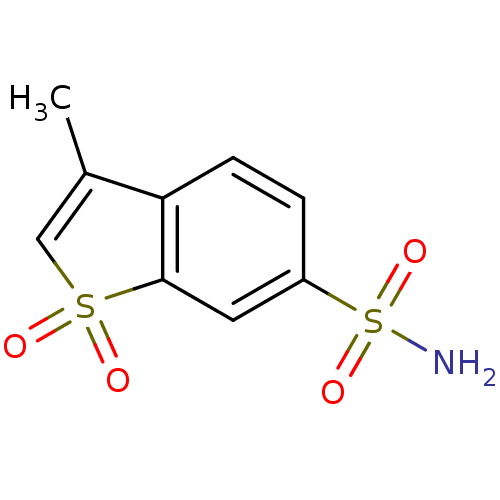
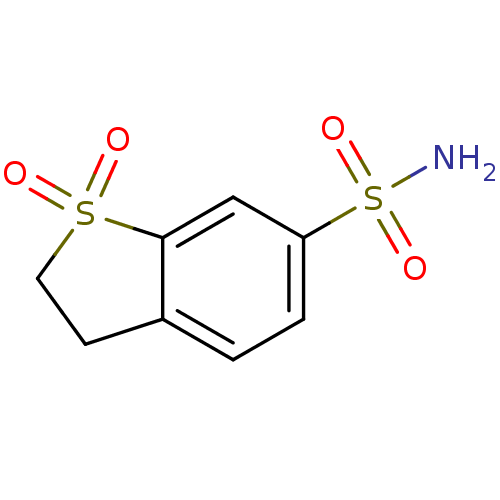
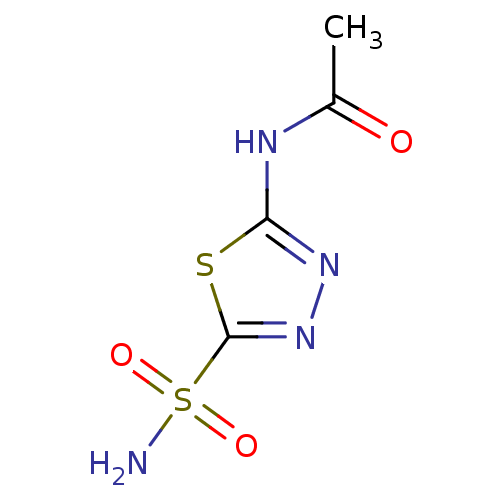
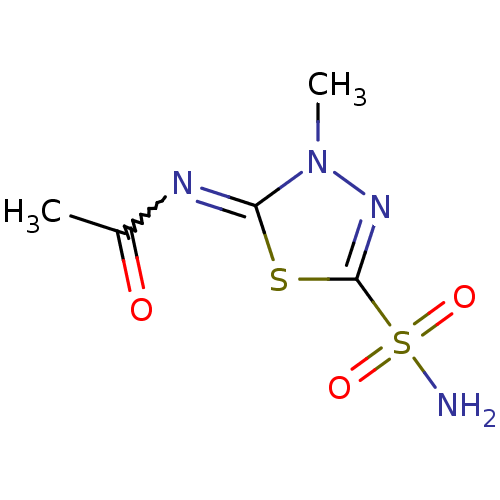
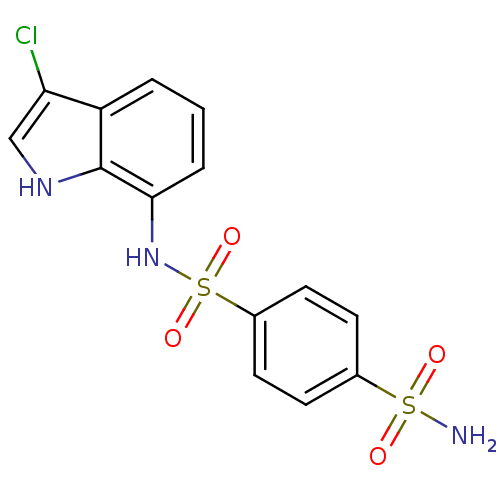
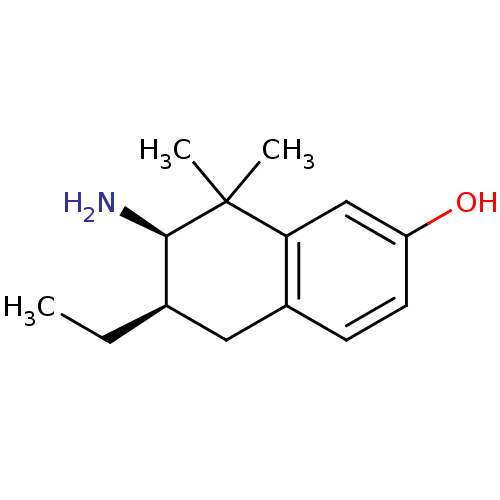
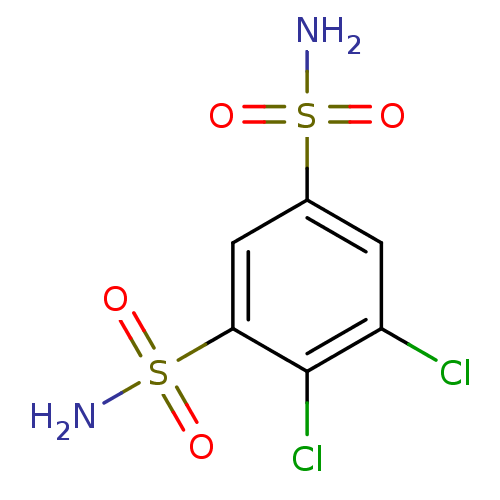
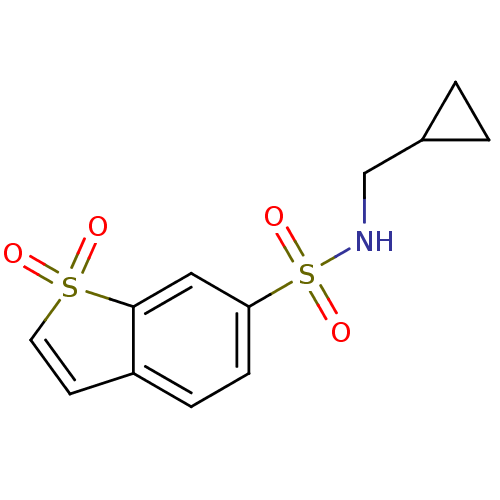
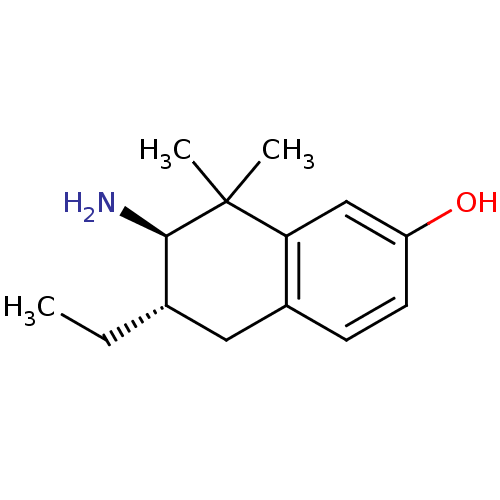
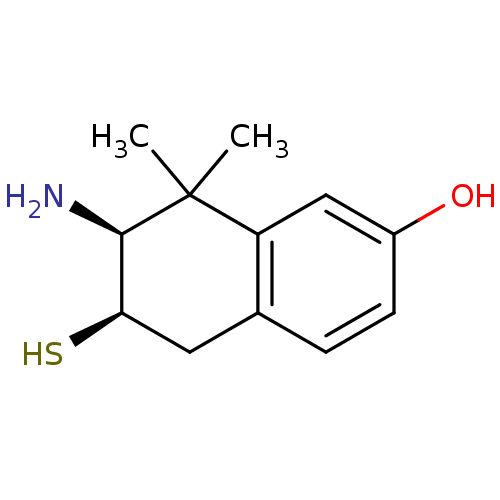
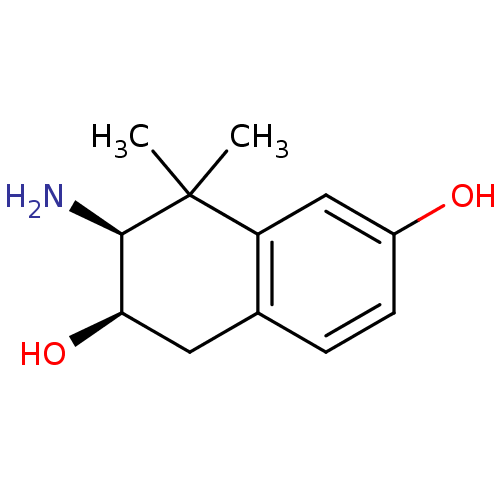
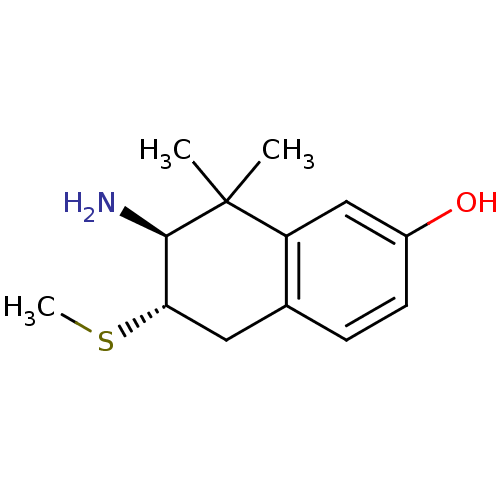
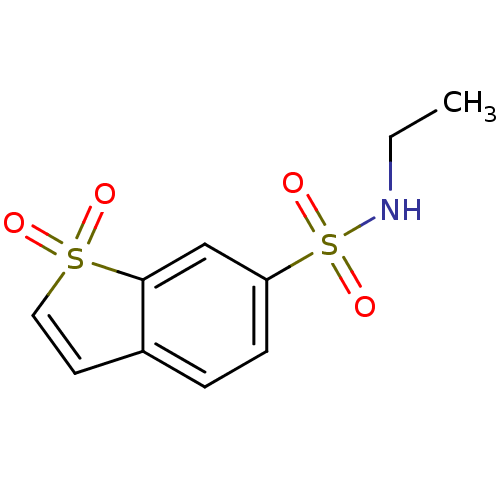
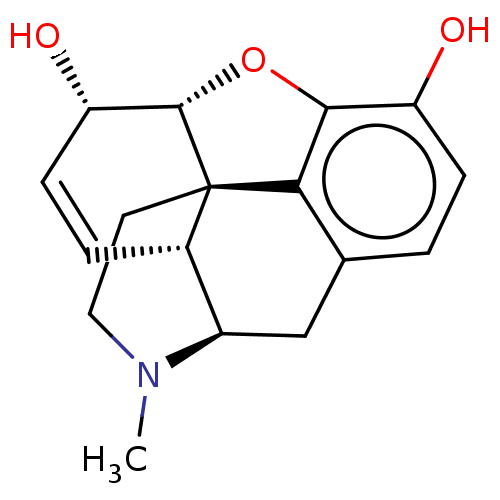
Affinity DataKi: 0.790nMAssay Description:Binding affinity for Opioid receptor mu 1More data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Binding affinity for Opioid receptor mu 1More data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Binding affinity for Opioid receptor mu 1More data for this Ligand-Target Pair
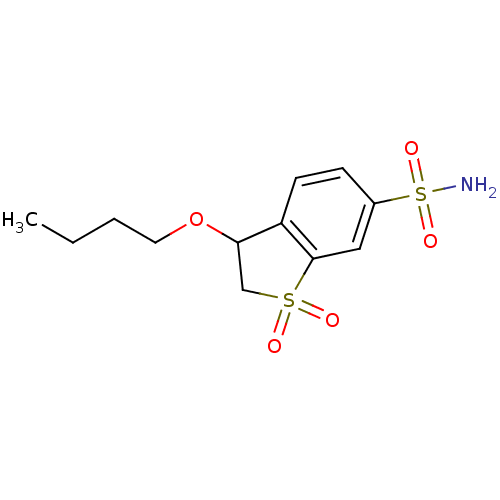
Affinity DataKi: 2.80nMAssay Description:Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 3.10nMAssay Description:Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Binding affinity for Opioid receptor mu 1More data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 7.40nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 7.5nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 7.90nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 8.30nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 8.80nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Binding affinity for Opioid receptor mu 1More data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase I by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 27nMAssay Description:Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 31nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase I by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 34nMAssay Description:Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 38nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Binding affinity of the compound for Opioid receptor kappa 1 was determined.More data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 52nMAssay Description:Binding affinity of the compound for Opioid receptor kappa 1 was determined.More data for this Ligand-Target Pair
Affinity DataKi: 63nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase I by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 66nMAssay Description:Binding affinity of the compound for Opioid receptor kappa 1 was determined.More data for this Ligand-Target Pair
Affinity DataKi: 72nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase I by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 75nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase I by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 76nMAssay Description:Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 85nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase I by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 104nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase I by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 113nMAssay Description:Binding affinity of the compound for Opioid receptor delta 1 was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 135nMAssay Description:Binding affinity for Opioid receptor mu 1More data for this Ligand-Target Pair
Affinity DataKi: 138nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase I by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 163nMAssay Description:Binding affinity for Opioid receptor mu 1More data for this Ligand-Target Pair
Affinity DataKi: 184nMAssay Description:Binding affinity of the compound for Opioid receptor kappa 1 was determined.More data for this Ligand-Target Pair
Affinity DataKi: 204nMAssay Description:Binding affinity for Opioid receptor mu 1More data for this Ligand-Target Pair
Affinity DataKi: 286nMAssay Description:Binding affinity for Opioid receptor mu 1More data for this Ligand-Target Pair
Affinity DataKi: 345nMAssay Description:Binding affinity of the compound for Opioid receptor kappa 1 was determined.More data for this Ligand-Target Pair
Affinity DataKi: 349nMAssay Description:Binding affinity of the compound for Opioid receptor delta 1 was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 362nMAssay Description:Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 420nMAssay Description:Binding affinity of the compound for Opioid receptor delta 1 was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 436nMAssay Description:Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration methodMore data for this Ligand-Target Pair

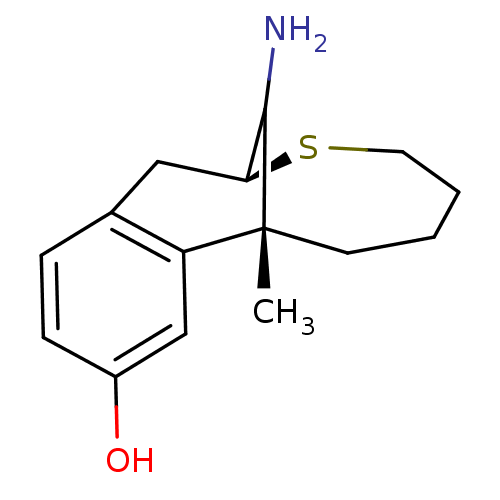
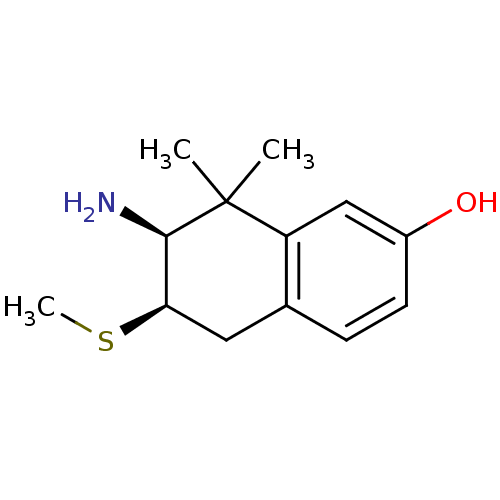
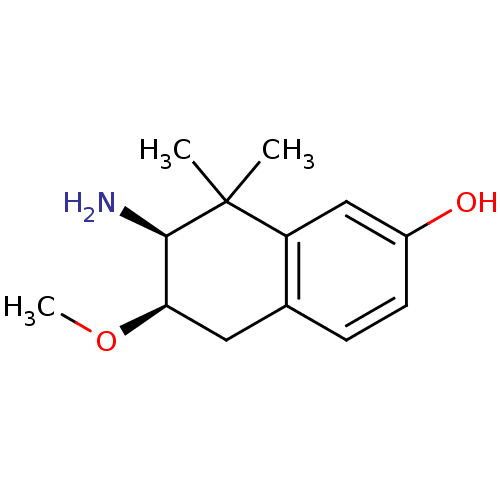
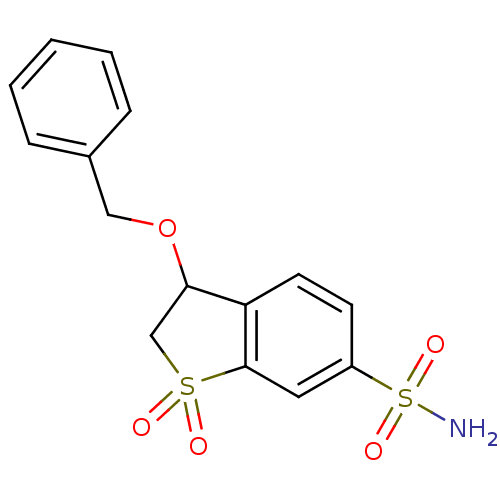
 3D Structure (crystal)
3D Structure (crystal)