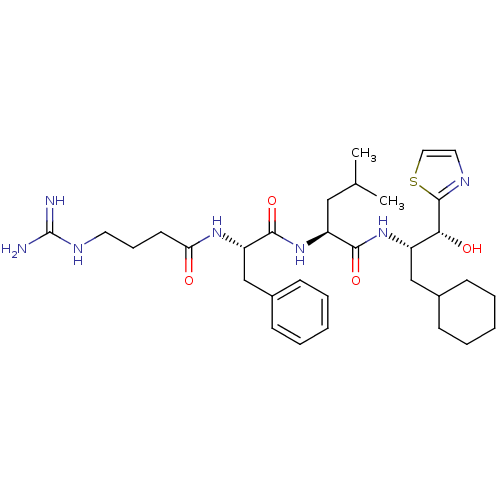
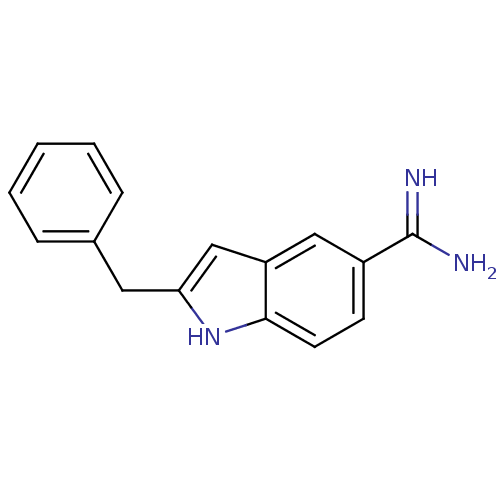
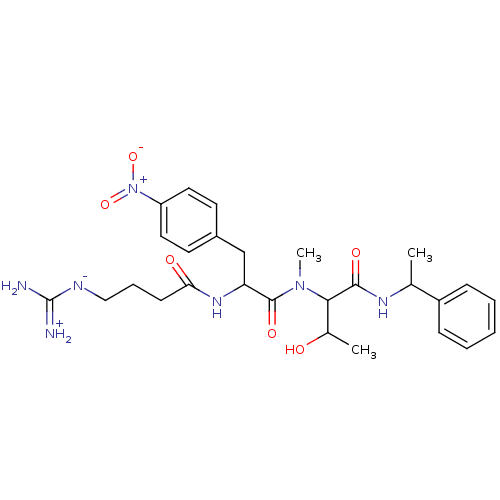
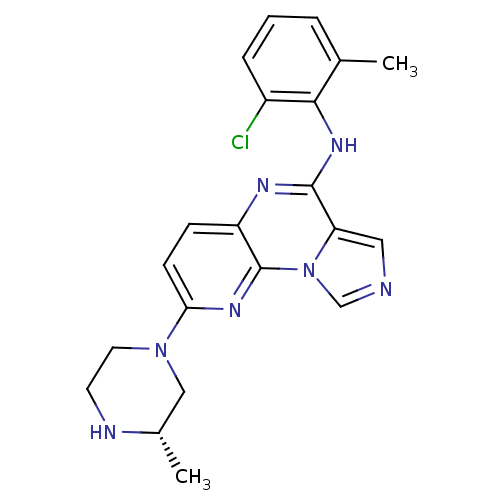
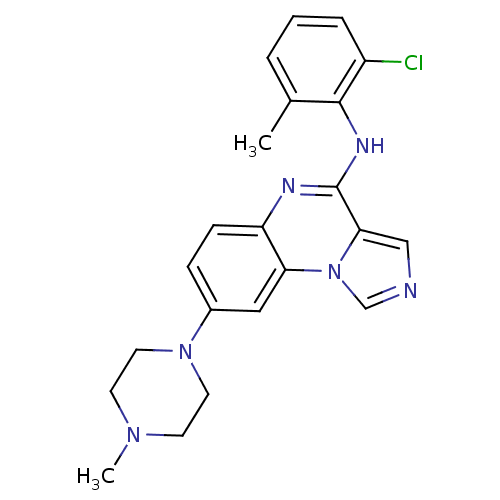
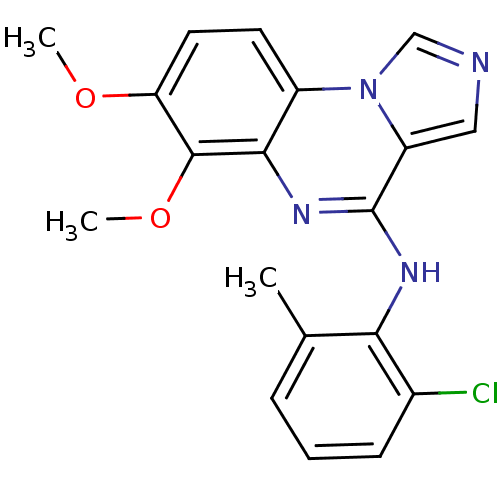
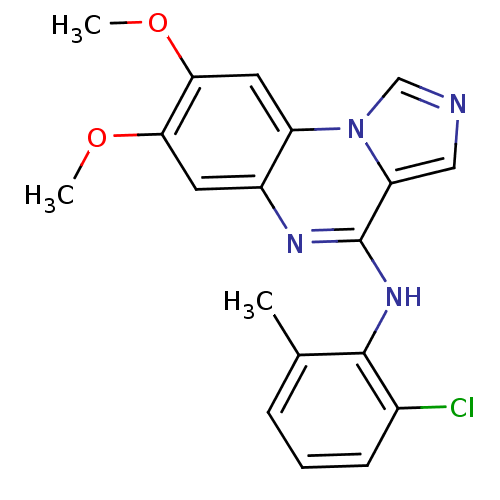
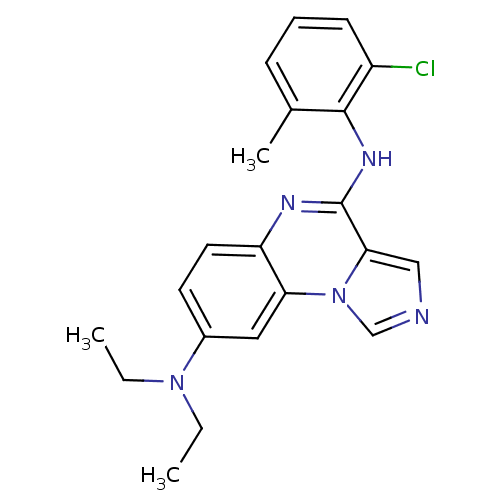
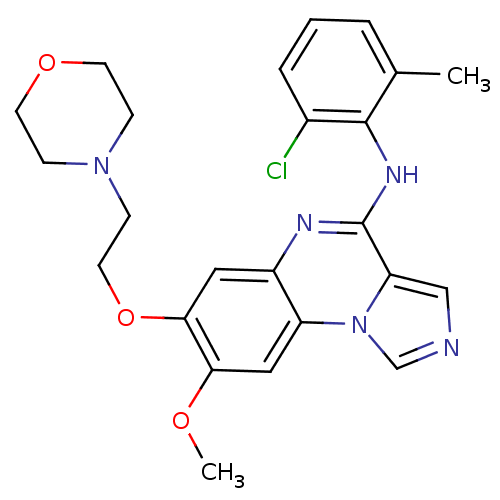
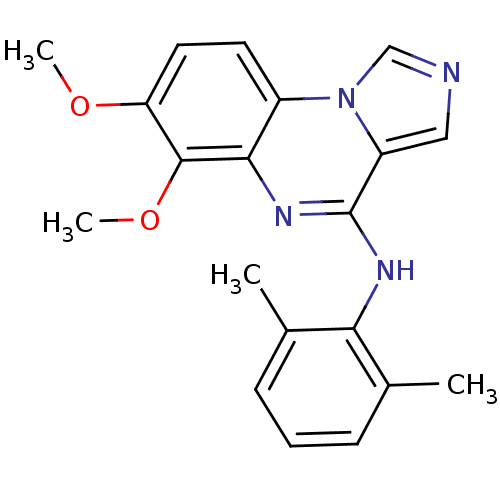
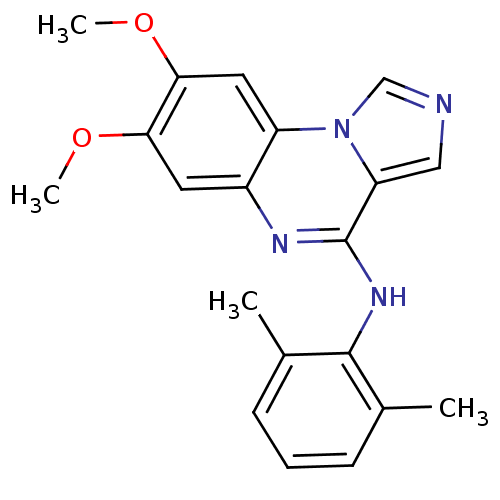
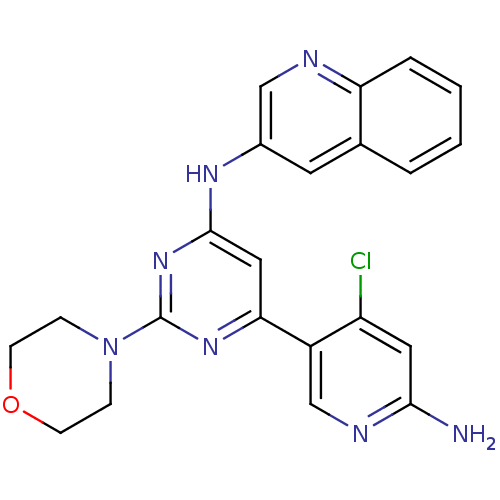
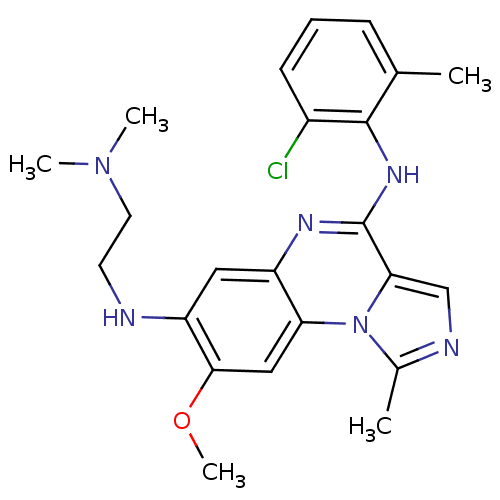
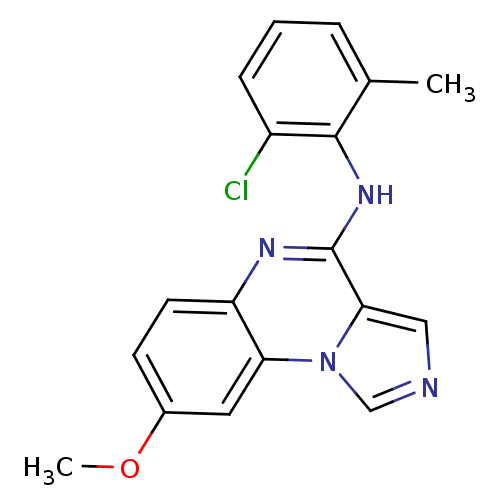
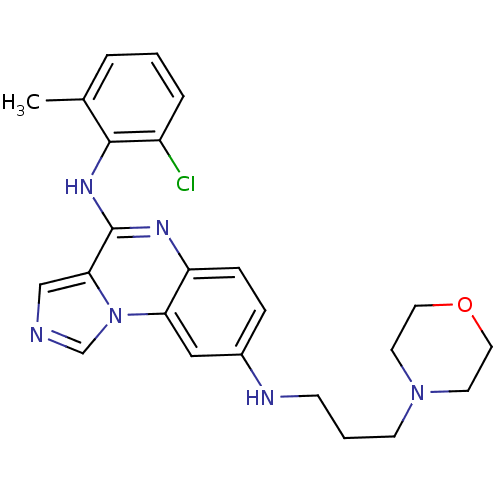
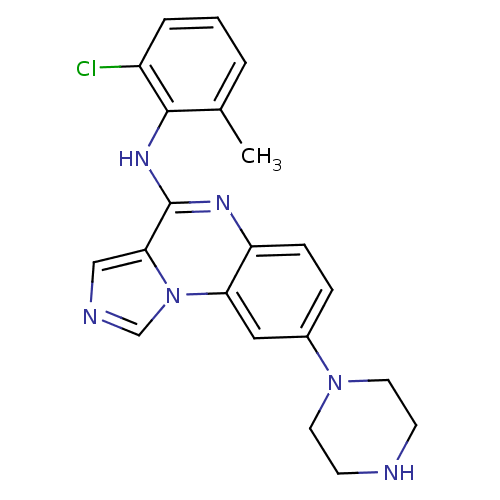
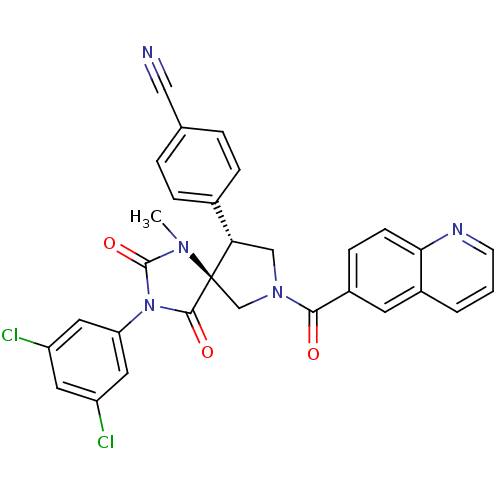
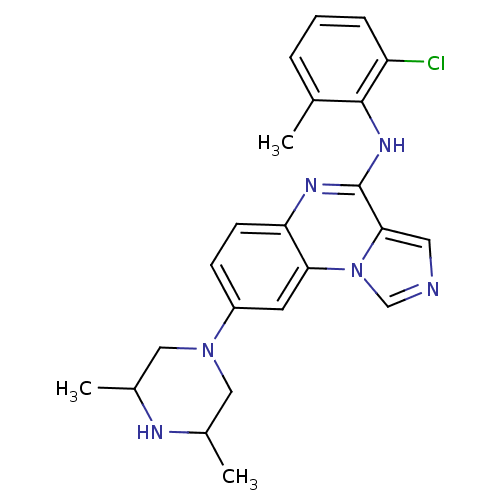
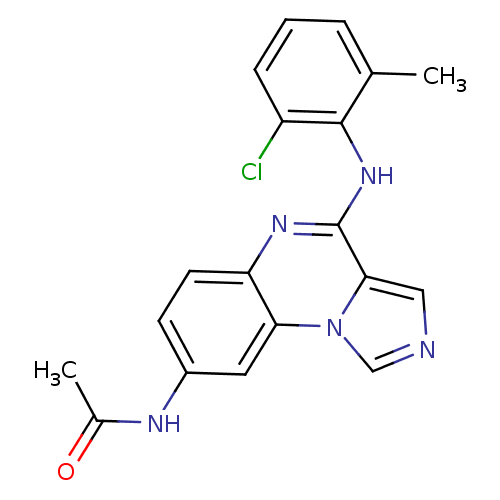
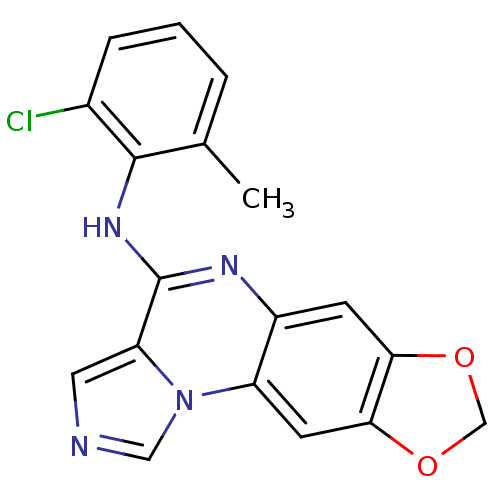
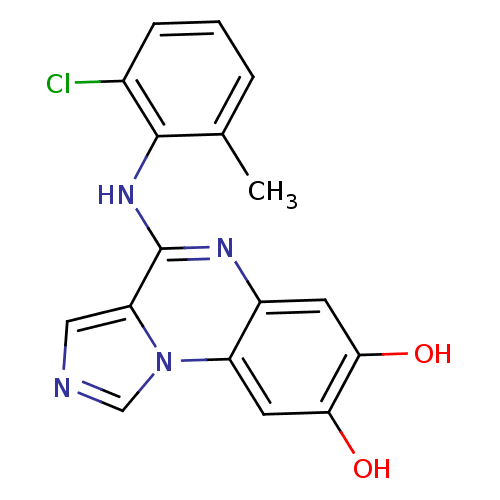
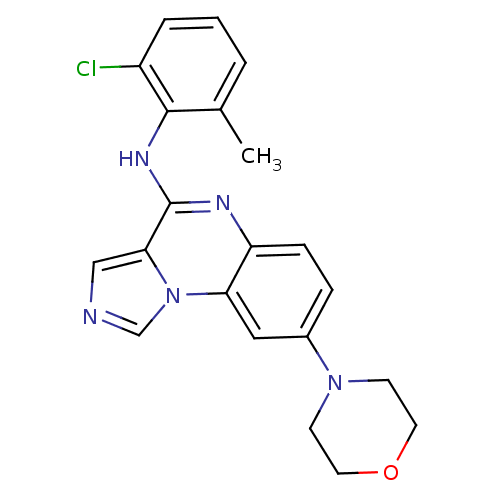
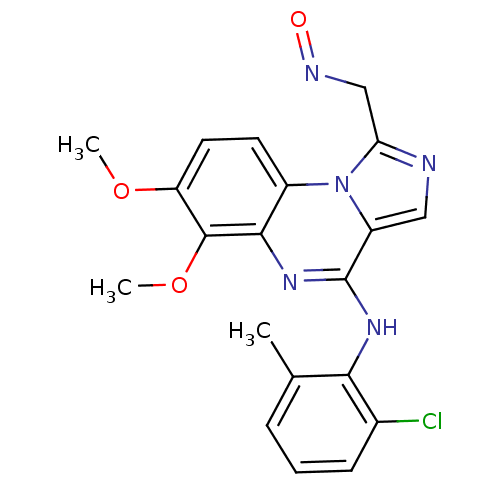
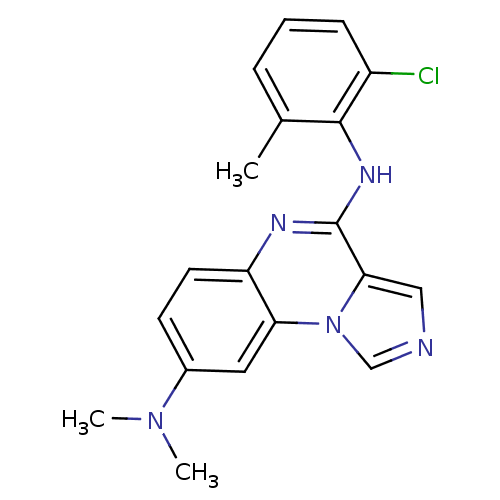
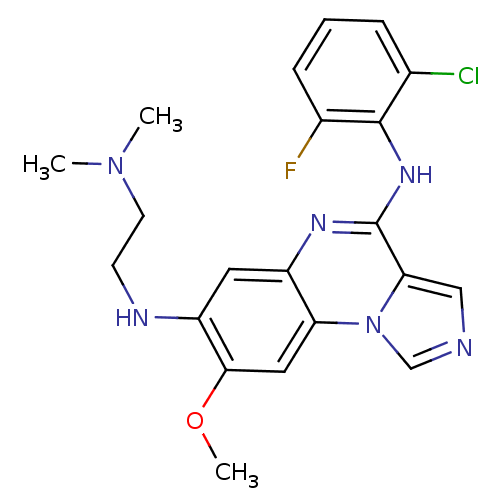
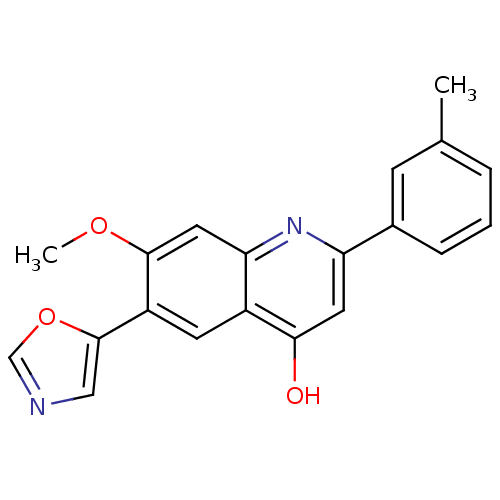
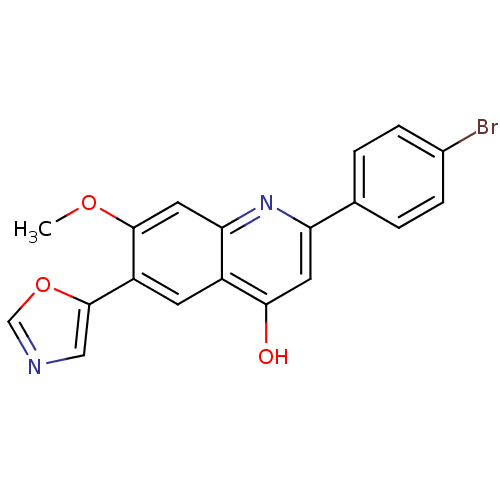
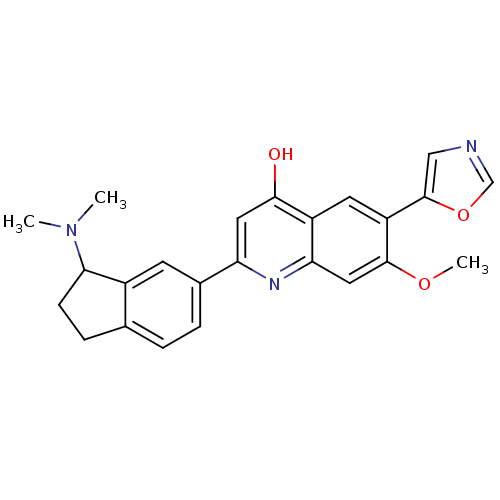
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.460nMAssay Description:Competitive kinetic for thrombin inhibition Ki was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Overall Inhibitory constant of the compound against thrombin was determinedMore data for this Ligand-Target Pair
TargetInosine-5'-monophosphate dehydrogenase 2(Homo sapiens (Human))
Bristol-Myers Squibb Pri
Curated by ChEMBL
Bristol-Myers Squibb Pri
Curated by ChEMBL
Affinity DataKi: 7nMAssay Description:Inhibitory activity of the compound against IMPDH II with respect to IMP and NADMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 7nMAssay Description:Concentration of the compound required to inhibit Human alpha-thrombin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 8.20nMAssay Description:Competitive kinetic for human alpha thrombin inhibition Ki was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 22nMAssay Description:Binding affinity for thrombin was reportedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 260nMAssay Description:Compound was evaluated for inhibition of human alpha-thrombin catalytic activityMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.00800nMAssay Description:Concentration of the compound required to inhibit thrombin was determinedMore data for this Ligand-Target Pair
TargetSerine protease 1(Bos taurus (bovine))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.00800nMAssay Description:Concentration of the compound required to inhibit Trypsin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.0300nMAssay Description:Concentration of the compound required to inhibit thrombin was determinedMore data for this Ligand-Target Pair
TargetSerine protease 1(Bos taurus (bovine))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.0450nMAssay Description:Concentration of the compound required to inhibit Trypsin was determinedMore data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.230nMAssay Description:Concentration of the compound required to inhibit Plasmin was determinedMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lyn(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: >0.5nMAssay Description:Inhibition of Lyn kinaseMore data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Concentration of the compound required to inhibit tissue-type plasminogen activator (t-PA) was determinedMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme.More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme.More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibitory activity against recombinant p56 Lck tyrosine kinase expressed as a His-tagged protein in insect cells using a baculovirus expression syst...More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme.More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme.More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:In vitro inhibitory activity against hydrolysis of thrombin was determinedMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 2.20nMAssay Description:Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assayMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme.More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Inhibitory activity against recombinant p56 Lck tyrosine kinase expressed as a His-tagged protein in insect cells using a baculovirus expression syst...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme.More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 2.60nMAssay Description:Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assayMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: >2.80nMAssay Description:Inhibition of Fgr protein kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme.More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme.More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme.More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme.More data for this Ligand-Target Pair
TargetIntegrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1(Homo sapiens (Human))
Cerep
Curated by ChEMBL
Cerep
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of LFA1-mediated adhesion of T cell to HUVECMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme.More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme.More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme.More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: >4nMAssay Description:Inhibition of Src kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme.More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 4.10nMAssay Description:Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assayMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.30nMAssay Description:Inhibitory activity against recombinant p56 Lck tyrosine kinase expressed as a His-tagged protein in insect cells using a baculovirus expression syst...More data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.60nMAssay Description:Concentration of the compound required to inhibit Coagulation factor X was determinedMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Yes(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: >4.70nMAssay Description:Inhibition of Yes kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme.More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme.More data for this Ligand-Target Pair
TargetInosine-5'-monophosphate dehydrogenase 2(Homo sapiens (Human))
Bristol-Myers Squibb Pri
Curated by ChEMBL
Bristol-Myers Squibb Pri
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human inosine monophosphate dehydrogenase IMPDH IIMore data for this Ligand-Target Pair
TargetInosine-5'-monophosphate dehydrogenase 2(Homo sapiens (Human))
Bristol-Myers Squibb Pri
Curated by ChEMBL
Bristol-Myers Squibb Pri
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human inosine monophosphate dehydrogenase IMPDH IIMore data for this Ligand-Target Pair
TargetInosine-5'-monophosphate dehydrogenase 2(Homo sapiens (Human))
Bristol-Myers Squibb Pri
Curated by ChEMBL
Bristol-Myers Squibb Pri
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibitory activity against inosine monophosphate dehydrogenase IMPDH IIMore data for this Ligand-Target Pair
TargetInosine-5'-monophosphate dehydrogenase 2(Homo sapiens (Human))
Bristol-Myers Squibb Pri
Curated by ChEMBL
Bristol-Myers Squibb Pri
Curated by ChEMBL
Affinity DataIC50: <5nMAssay Description:Inhibitory activity against inosine monophosphate dehydrogenase IMPDH IIMore data for this Ligand-Target Pair

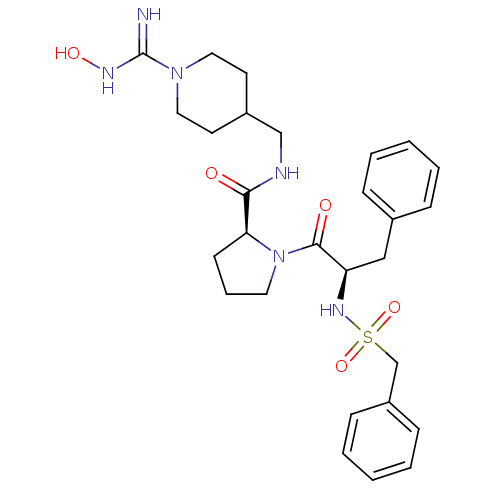

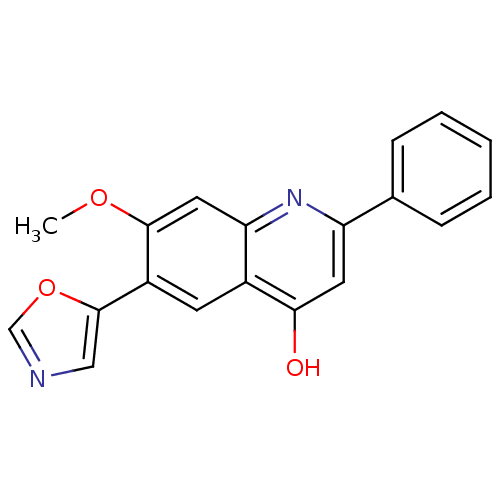
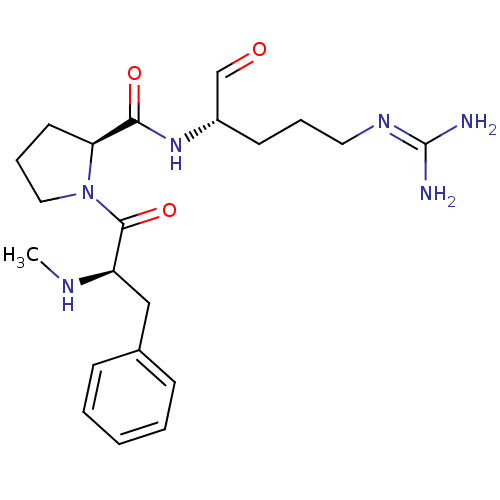
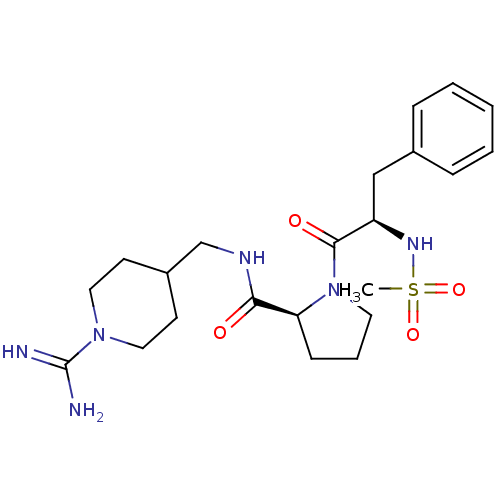
 3D Structure (crystal)
3D Structure (crystal)