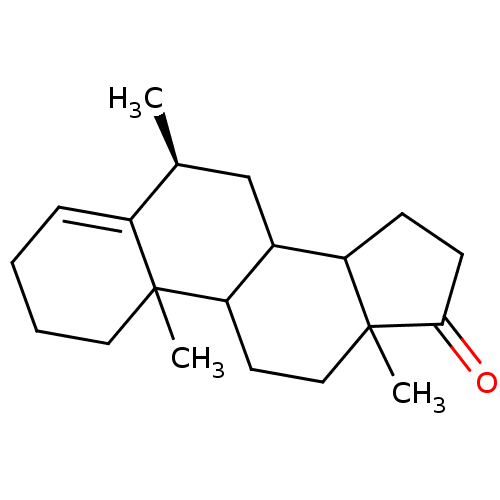
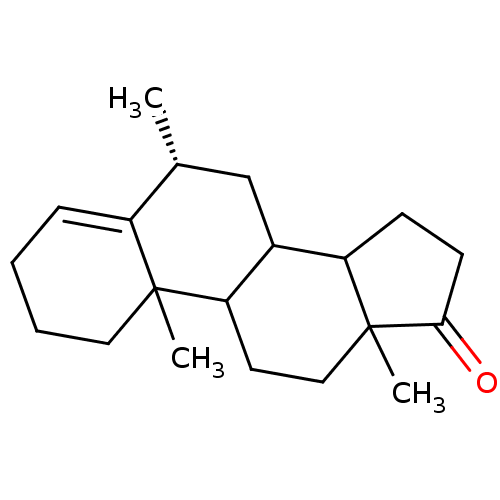
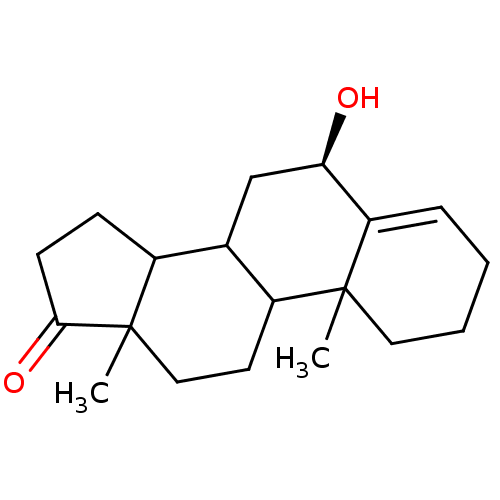
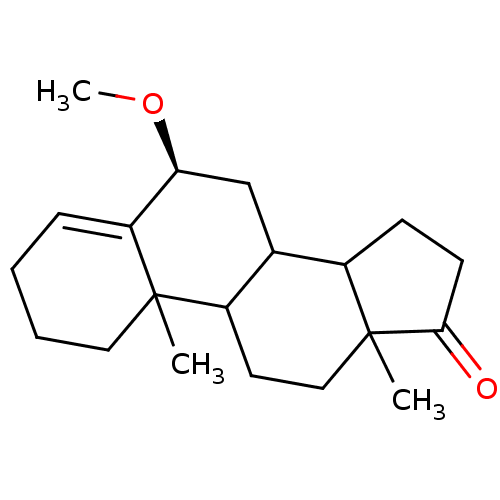
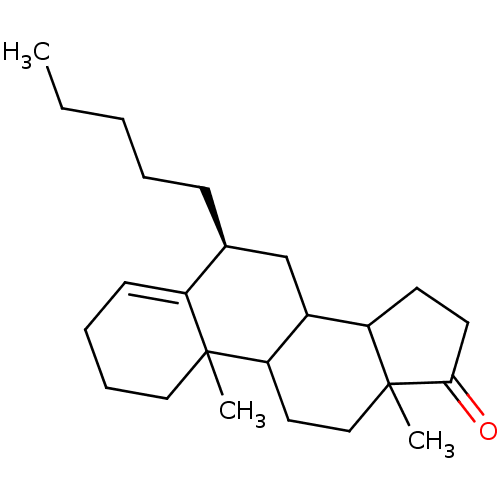
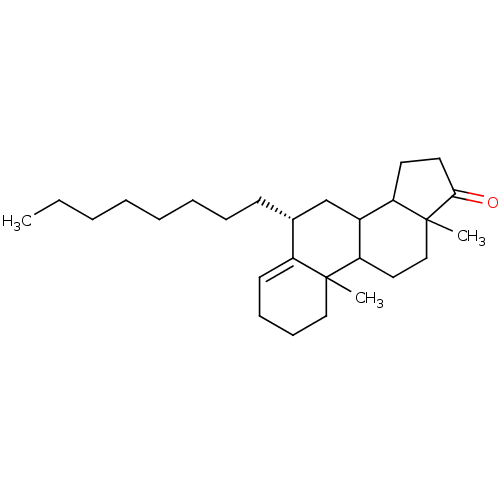
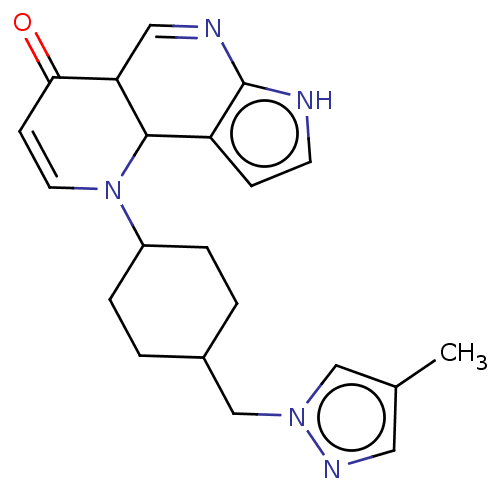
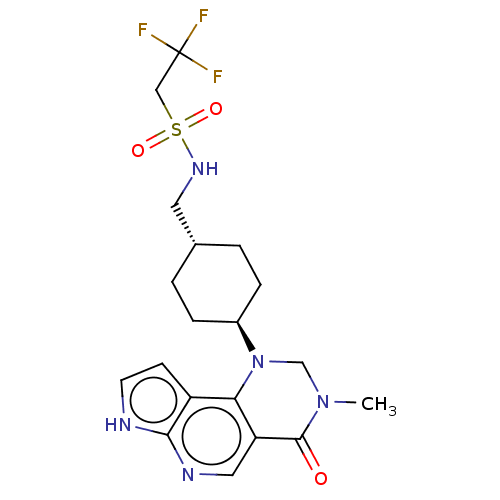
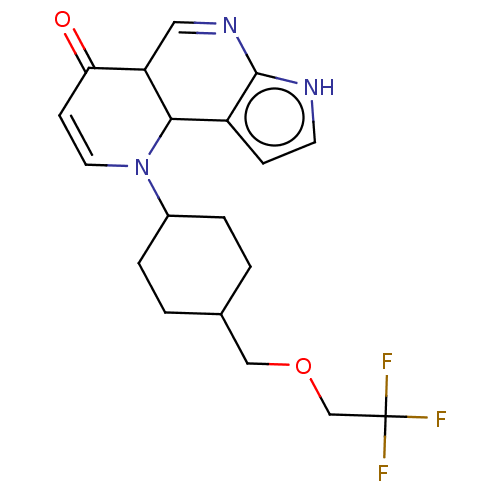
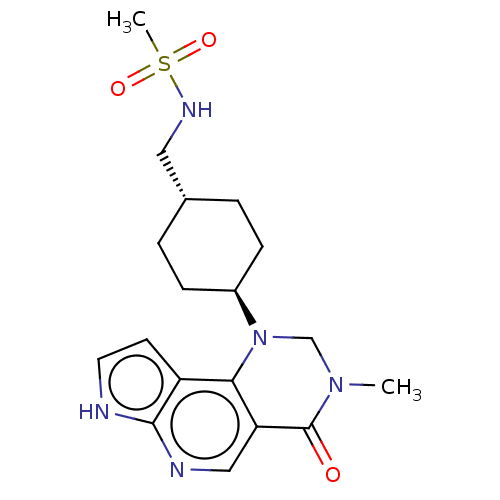
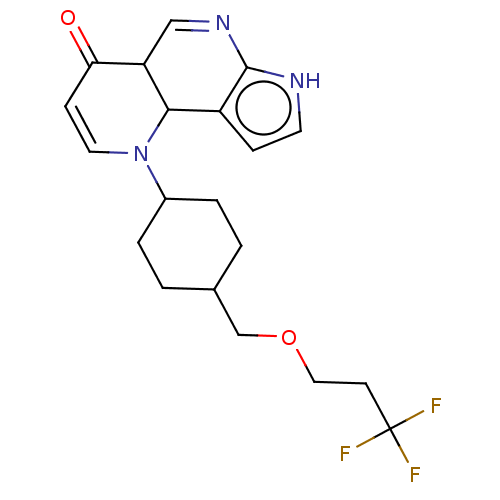
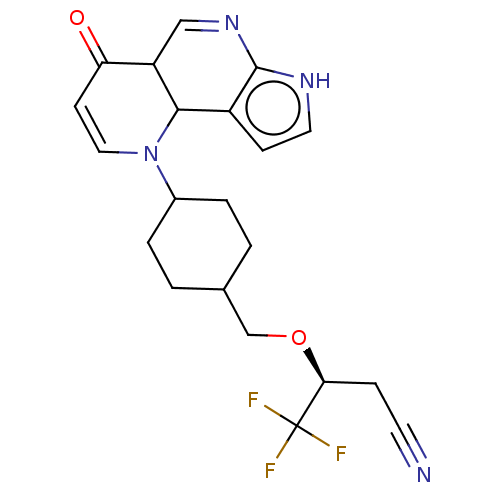
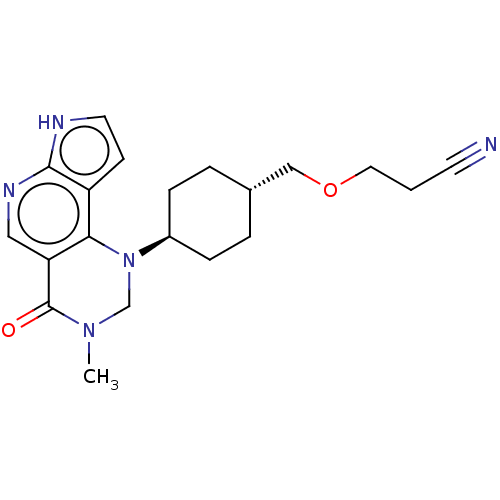
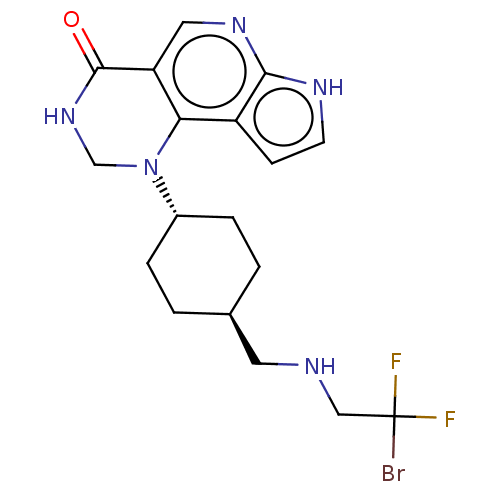
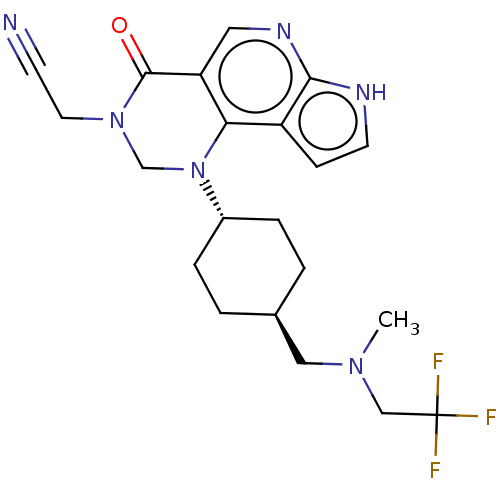
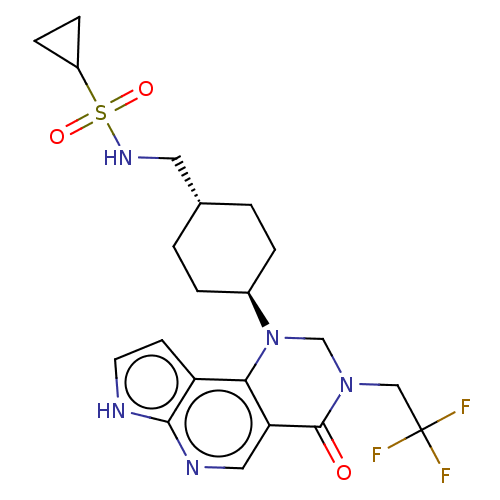
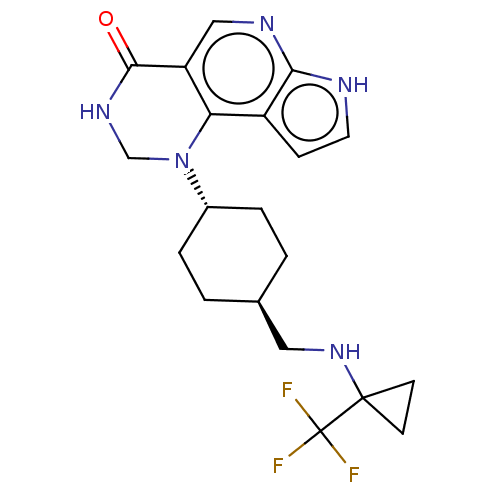
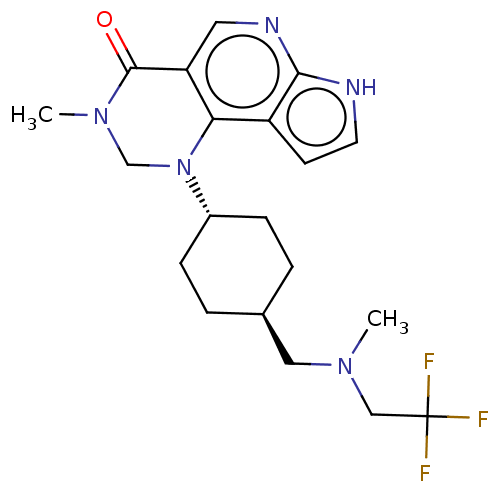
Affinity DataKi: 3.10nM ΔG°: -50.5kJ/mole IC50: 37nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
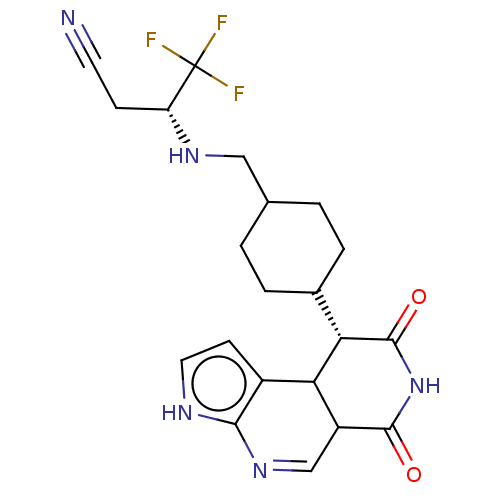
Affinity DataKi: 5.30nM ΔG°: -49.1kJ/mole IC50: 49nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
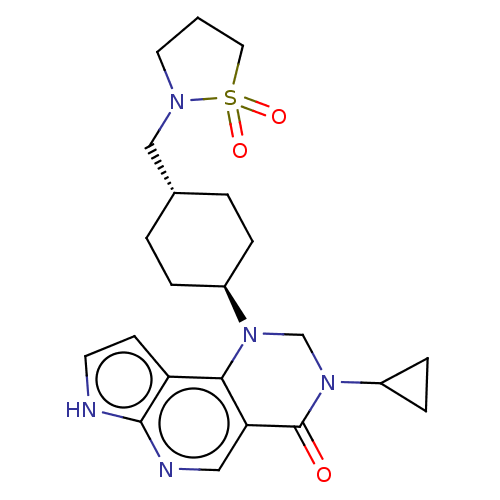
Affinity DataKi: 6nM ΔG°: -48.8kJ/mole IC50: 50nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
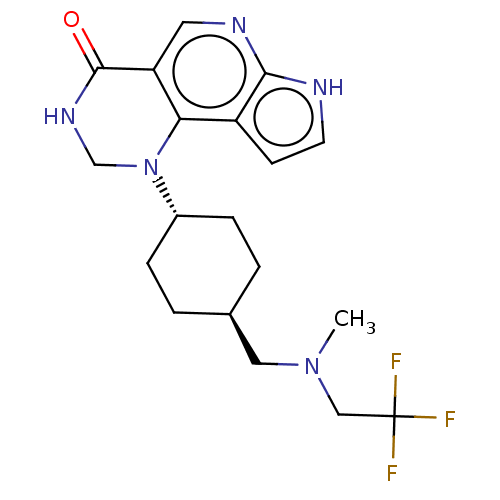
Affinity DataKi: 6.80nM IC50: 60nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 12nM ΔG°: -47.0kJ/mole IC50: 120nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 12nM IC50: 130nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 14nM ΔG°: -46.6kJ/mole IC50: 110nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 18nM ΔG°: -46.0kJ/mole IC50: 160nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Institute For Theoretical Medicine
Curated by ChEMBL
Institute For Theoretical Medicine
Curated by ChEMBL
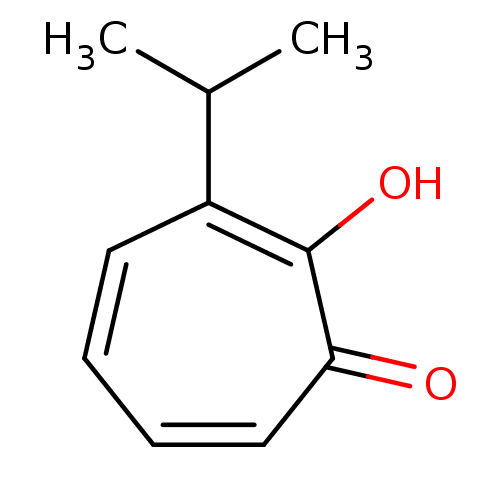
Affinity DataKi: 20nMAssay Description:Competitive inhibition of mushroom tyrosinase after 15 mins by Lineweaver-Bulk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 21nM ΔG°: -45.6kJ/mole IC50: 190nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 24nM IC50: 260nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 30nM ΔG°: -44.7kJ/mole IC50: 230nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 43nM ΔG°: -43.7kJ/mole IC50: 320nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 45nM IC50: 360nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 51nM ΔG°: -43.3kJ/mole IC50: 440nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Institute For Theoretical Medicine
Curated by ChEMBL
Institute For Theoretical Medicine
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Competitive inhibition of mushroom tyrosinase after 15 mins by Lineweaver-Bulk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 140nM IC50: 1.60E+3nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 180nM IC50: 1.30E+3nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 340nM IC50: 3.30E+3nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 380nM IC50: 3.70E+3nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 400nM ΔG°: -38.0kJ/mole IC50: 9.00E+3nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nM ΔG°: -35.2kJ/mole IC50: 1.20E+4nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 2.60E+3nM ΔG°: -33.2kJ/mole IC50: >5.00E+4nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 2.90E+3nM IC50: 1.40E+4nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Institute For Theoretical Medicine
Curated by ChEMBL
Institute For Theoretical Medicine
Curated by ChEMBL
Affinity DataKi: 3.30E+3nMAssay Description:Competitive inhibition of mushroom tyrosinase after 15 mins by Lineweaver-Bulk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 4.10E+3nM ΔG°: -32.0kJ/mole IC50: >5.00E+4nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 5.50E+3nM IC50: 4.30E+4nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 4.40E+4nM IC50: >5.00E+4nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+5nM IC50: >5.00E+4nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
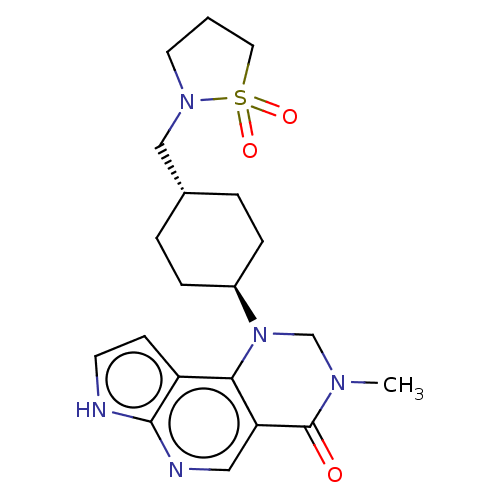
Affinity DataIC50: 0.350nMAssay Description:The JAK inhibitory activities of compounds of the present invention were measured.The enzymes (JAK1, JAK2, JAK3 and Tyk2) were purchased from Carna B...More data for this Ligand-Target Pair
Affinity DataIC50: 0.380nMAssay Description:The inhibitory activity of the compounds of the present invention against JAK was measured.The respective enzymes (JAK1, JAK2, JAK3 and Tyk2) were pu...More data for this Ligand-Target Pair
Affinity DataIC50: 0.410nMAssay Description:The JAK inhibitory activities of compounds of the present invention were measured.The enzymes (JAK1, JAK2, JAK3 and Tyk2) were purchased from Carna B...More data for this Ligand-Target Pair
Affinity DataIC50: 0.420nMAssay Description:The inhibitory activity of the compounds of the present invention against JAK was measured.The respective enzymes (JAK1, JAK2, JAK3 and Tyk2) were pu...More data for this Ligand-Target Pair
Affinity DataIC50: 0.590nMAssay Description:The JAK inhibitory activities of compounds of the present invention were measured.The enzymes (JAK1, JAK2, JAK3 and Tyk2) were purchased from Carna B...More data for this Ligand-Target Pair
Affinity DataIC50: 0.620nMAssay Description:The inhibitory activity of the compounds of the present invention against JAK was measured.The respective enzymes (JAK1, JAK2, JAK3 and Tyk2) were pu...More data for this Ligand-Target Pair
Affinity DataIC50: 0.650nMAssay Description:The inhibitory activity of the compounds of the present invention against JAK was measured.The respective enzymes (JAK1, JAK2, JAK3 and Tyk2) were pu...More data for this Ligand-Target Pair
Affinity DataIC50: 0.680nMAssay Description:The JAK inhibitory activities of compounds of the present invention were measured.The enzymes (JAK1, JAK2, JAK3 and Tyk2) were purchased from Carna B...More data for this Ligand-Target Pair
Affinity DataIC50: 0.740nMAssay Description:The JAK inhibitory activities of compounds of the present invention were measured.The enzymes (JAK1, JAK2, JAK3 and Tyk2) were purchased from Carna B...More data for this Ligand-Target Pair
Affinity DataIC50: 0.850nMAssay Description:The inhibitory activity of the compounds of the present invention against JAK was measured.The respective enzymes (JAK1, JAK2, JAK3 and Tyk2) were pu...More data for this Ligand-Target Pair
Affinity DataIC50: 0.880nMAssay Description:The inhibitory activity of the compounds of the present invention against JAK was measured.The respective enzymes (JAK1, JAK2, JAK3 and Tyk2) were pu...More data for this Ligand-Target Pair
Affinity DataIC50: 0.880nMAssay Description:The inhibitory activity of the compounds of the present invention against JAK was measured.The respective enzymes (JAK1, JAK2, JAK3 and Tyk2) were pu...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The inhibitory activity of the compounds of the present invention against JAK was measured.The respective enzymes (JAK1, JAK2, JAK3 and Tyk2) were pu...More data for this Ligand-Target Pair
Affinity DataIC50: 0.990nMAssay Description:The JAK inhibitory activities of compounds of the present invention were measured.The enzymes (JAK1, JAK2, JAK3 and Tyk2) were purchased from Carna B...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:The inhibitory activity of the compounds of the present invention against JAK was measured.The respective enzymes (JAK1, JAK2, JAK3 and Tyk2) were pu...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:The inhibitory activity of the compounds of the present invention against JAK was measured.The respective enzymes (JAK1, JAK2, JAK3 and Tyk2) were pu...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:The inhibitory activity of the compounds of the present invention against JAK was measured.The respective enzymes (JAK1, JAK2, JAK3 and Tyk2) were pu...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:The inhibitory activity of the compounds of the present invention against JAK was measured.The respective enzymes (JAK1, JAK2, JAK3 and Tyk2) were pu...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:The inhibitory activity of the compounds of the present invention against JAK was measured.The respective enzymes (JAK1, JAK2, JAK3 and Tyk2) were pu...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:The JAK inhibitory activities of compounds of the present invention were measured.The enzymes (JAK1, JAK2, JAK3 and Tyk2) were purchased from Carna B...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:The JAK inhibitory activities of compounds of the present invention were measured.The enzymes (JAK1, JAK2, JAK3 and Tyk2) were purchased from Carna B...More data for this Ligand-Target Pair