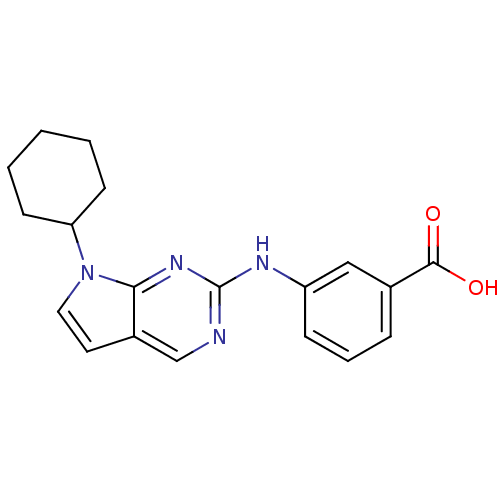
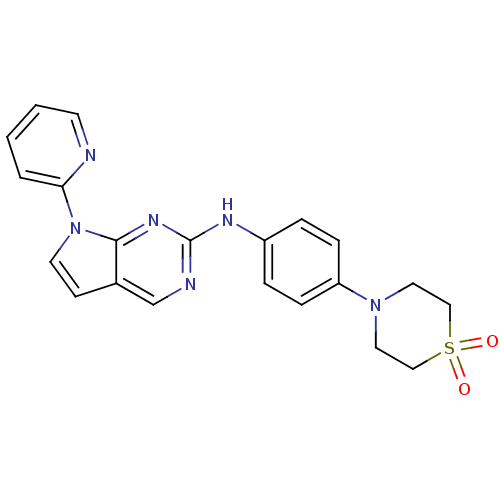
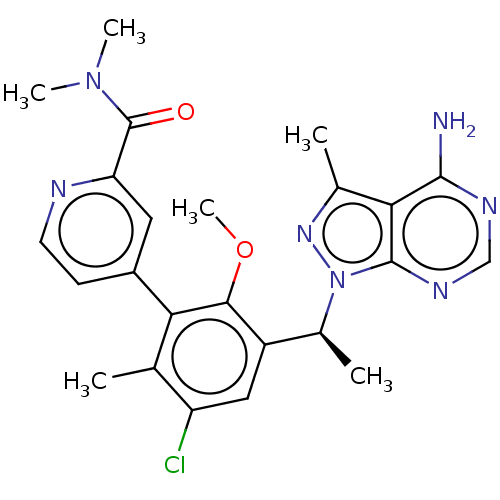
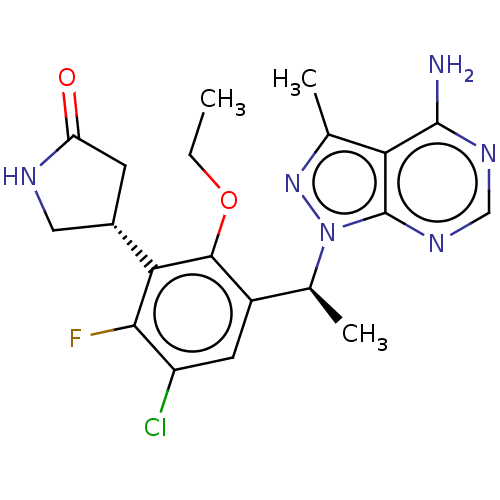
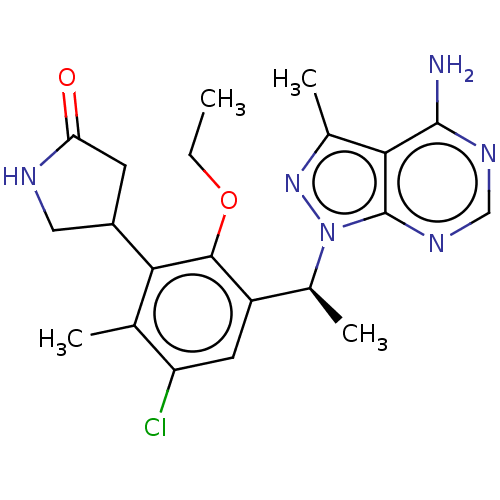
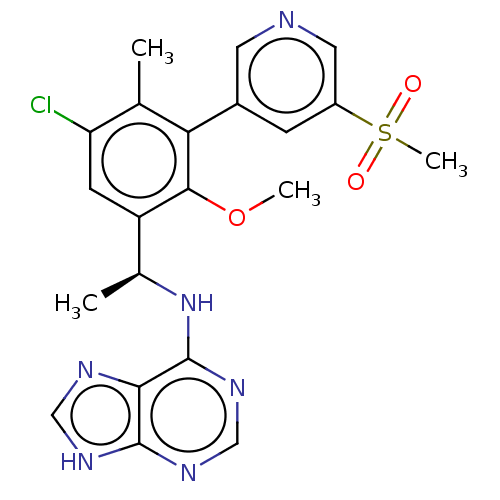
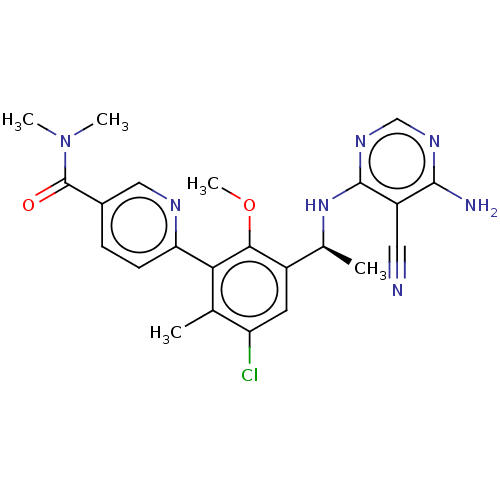
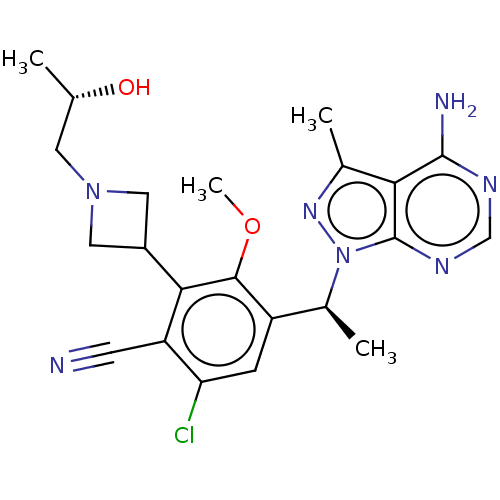
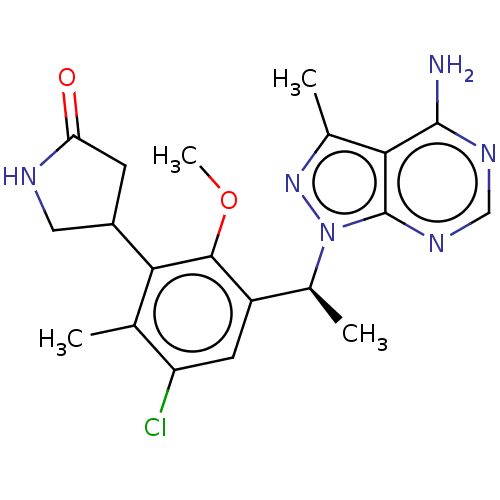
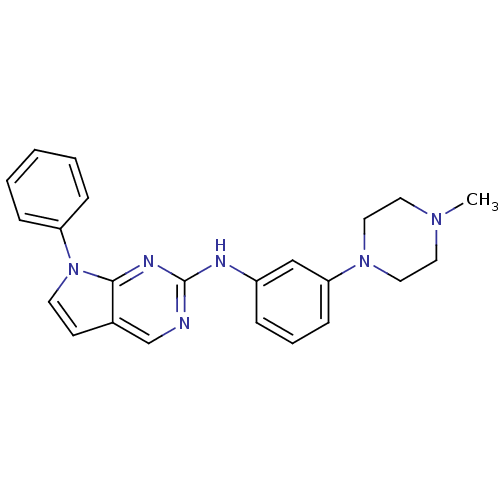
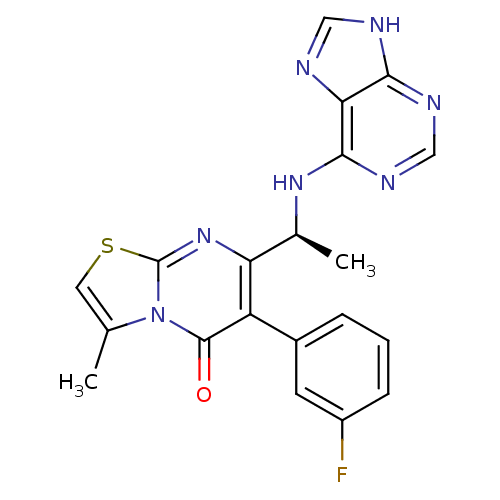
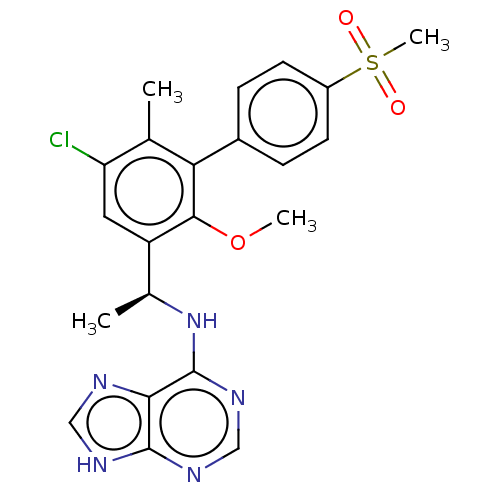
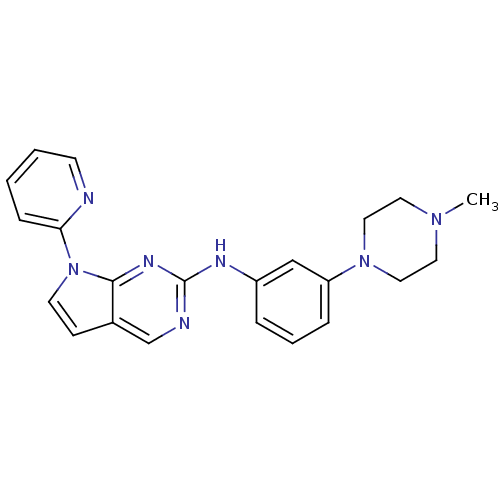
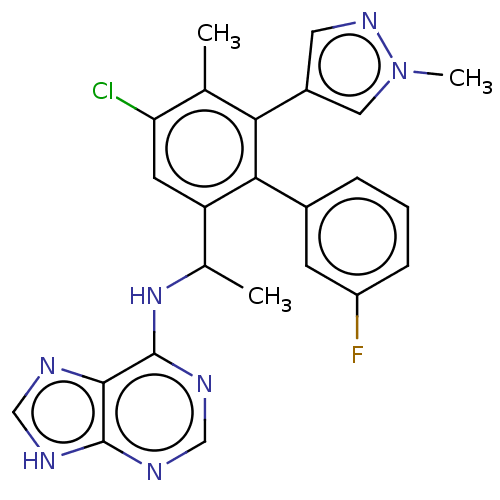
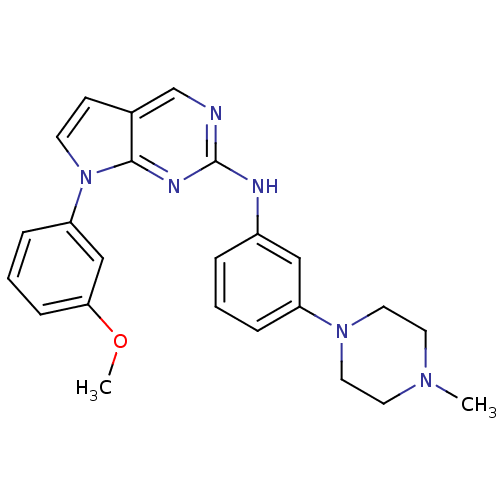
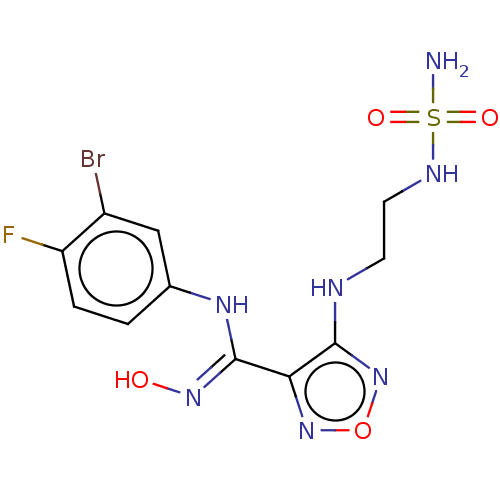
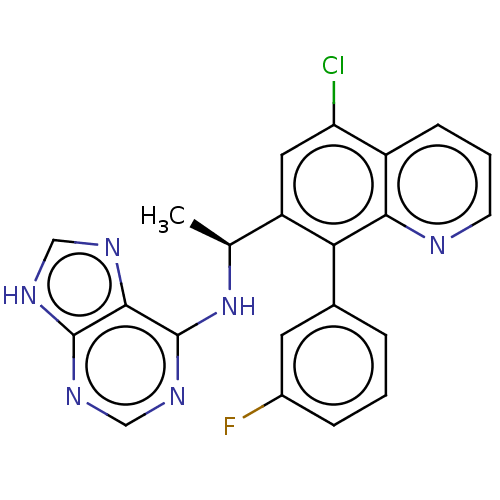
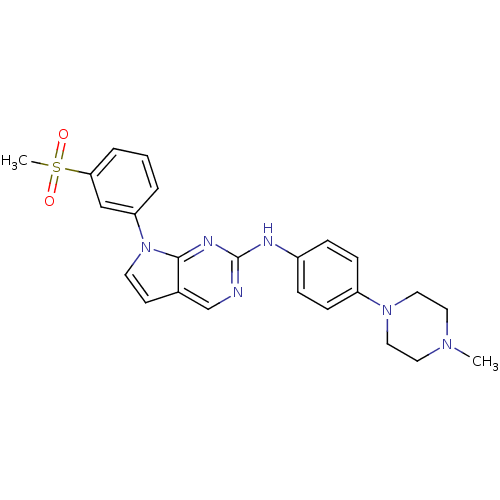
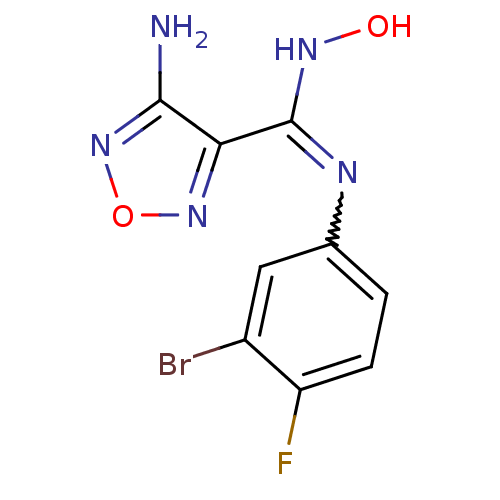
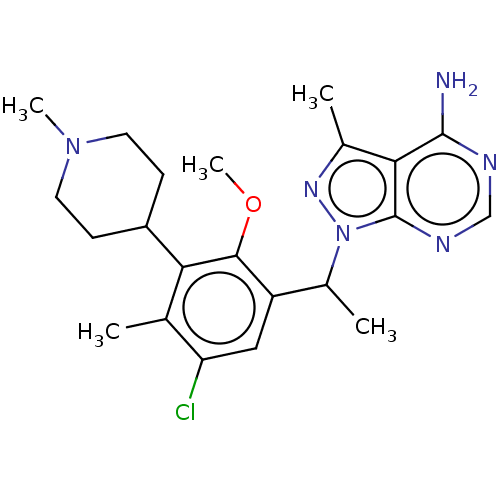
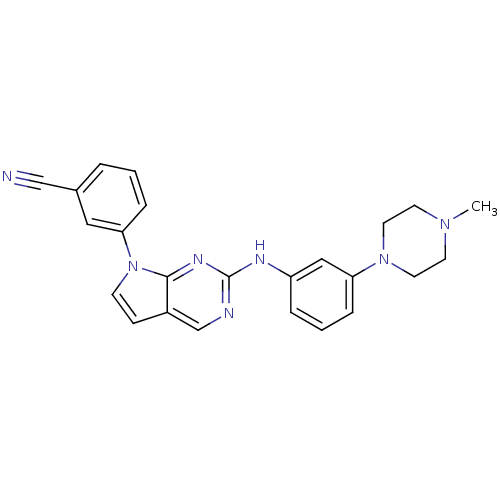
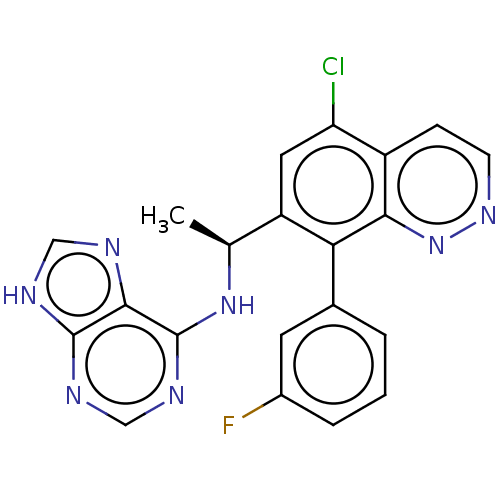
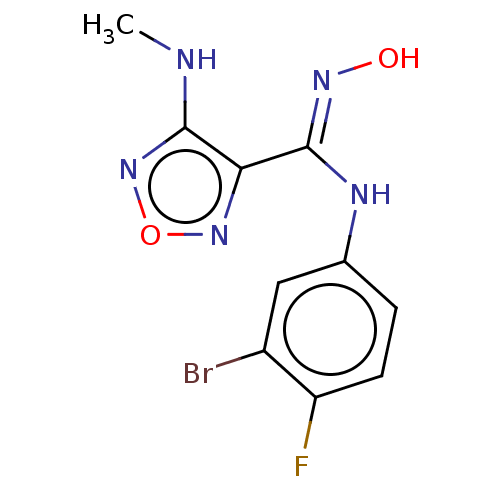
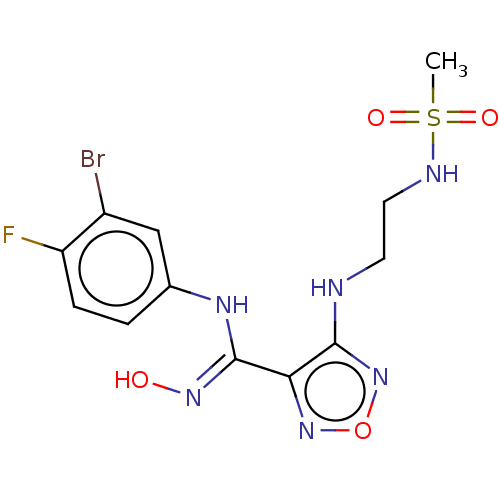
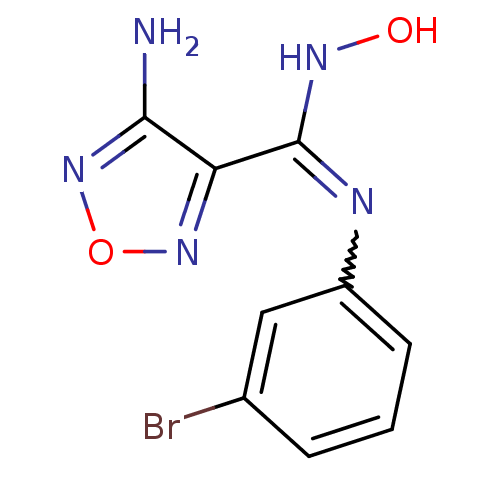
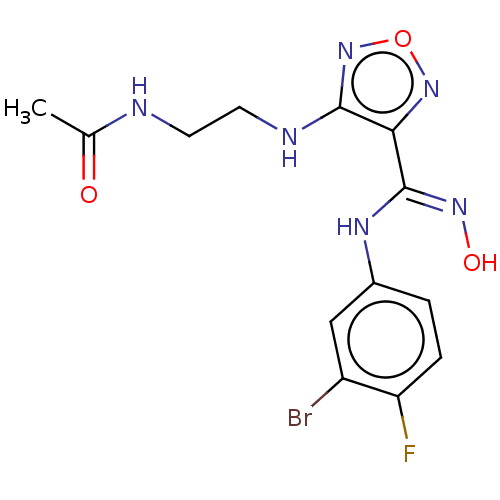
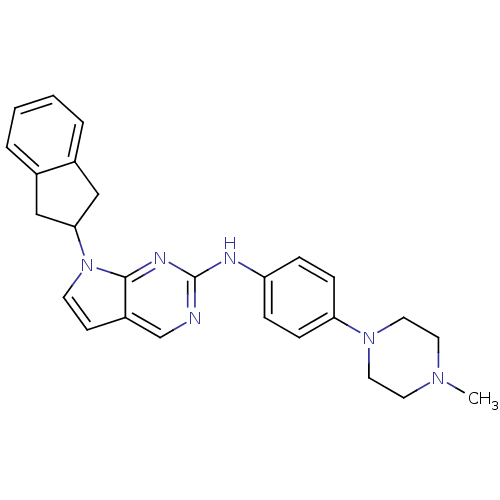
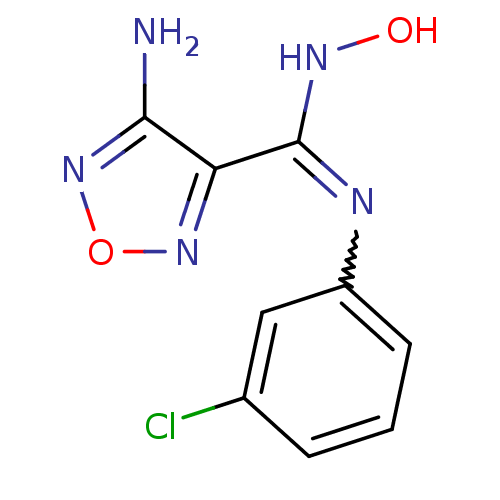
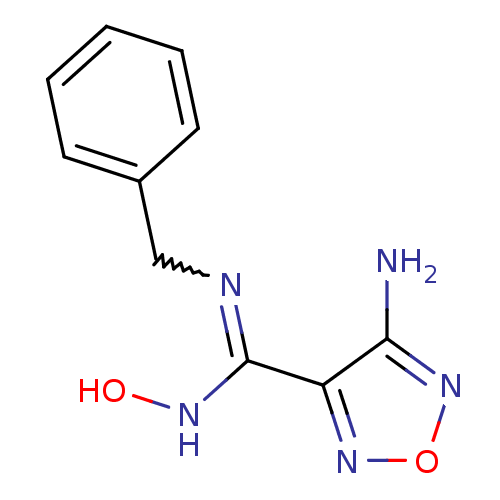
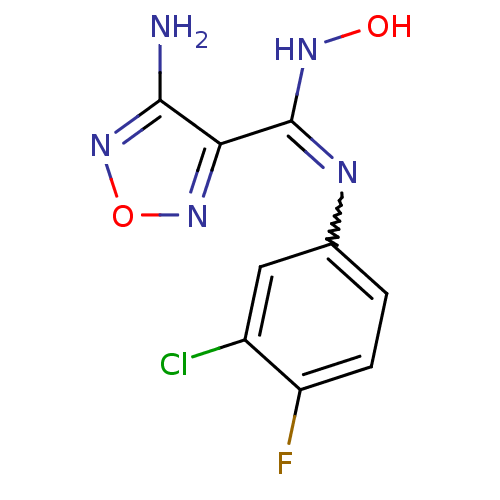
Affinity DataKi: 1.10E+3nMAssay Description:Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec...More data for this Ligand-Target Pair
Affinity DataKi: 3.40E+4nMAssay Description:Competitive inhibition of IDO1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Incyte
Curated by ChEMBL
Incyte
Curated by ChEMBL
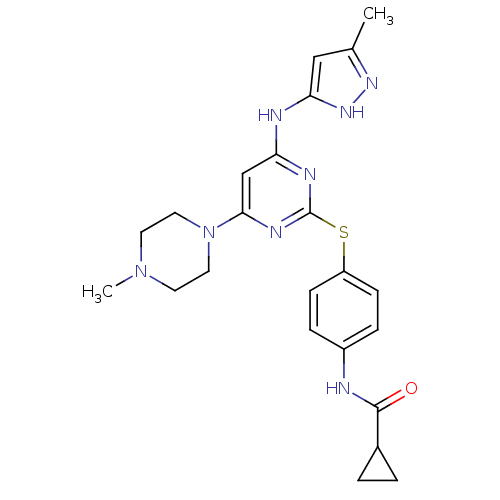
Affinity DataIC50: 0.930nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Incyte
Curated by ChEMBL
Incyte
Curated by ChEMBL
Affinity DataIC50: 0.990nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Incyte
Curated by ChEMBL
Incyte
Curated by ChEMBL
Affinity DataIC50: <1nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Incyte
Curated by ChEMBL
Incyte
Curated by ChEMBL
Affinity DataIC50: <1nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of poly(Glu:Tyr) by purified recombinant human FLT3. The extent of phospho...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Incyte
Curated by ChEMBL
Incyte
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of PI3Kdelta in human Ramos cells assessed as reduction in AKT phosphorylation incubated for 2 hrs by Alexa flour 488 based FACS analysisMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Incyte
Curated by ChEMBL
Incyte
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]-ATP base...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Incyte
Curated by ChEMBL
Incyte
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Incyte
Curated by ChEMBL
Incyte
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Incyte
Curated by ChEMBL
Incyte
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Incyte
Curated by ChEMBL
Incyte
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Incyte
Curated by ChEMBL
Incyte
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of PI3Kdelta in human whole blood assessed as reduction in anti-IgE antibody-induced CD63 expression by flow cast kit methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Incyte
Curated by ChEMBL
Incyte
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Incyte
Curated by ChEMBL
Incyte
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Incyte
Curated by ChEMBL
Incyte
Curated by ChEMBL
Affinity DataIC50: 3.40nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Incyte
Curated by ChEMBL
Incyte
Curated by ChEMBL
Affinity DataIC50: 3.60nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Incyte
Curated by ChEMBL
Incyte
Curated by ChEMBL
Affinity DataIC50: 5.30nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
Affinity DataIC50: 6nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
Affinity DataIC50: 7.40nMAssay Description:Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrsMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Incyte
Curated by ChEMBL
Incyte
Curated by ChEMBL
Affinity DataIC50: 8.40nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Incyte
Curated by ChEMBL
Incyte
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
Affinity DataIC50: 12nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
Affinity DataIC50: 13nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
Affinity DataIC50: 13nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Incyte
Curated by ChEMBL
Incyte
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
Affinity DataIC50: 18nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometryMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)