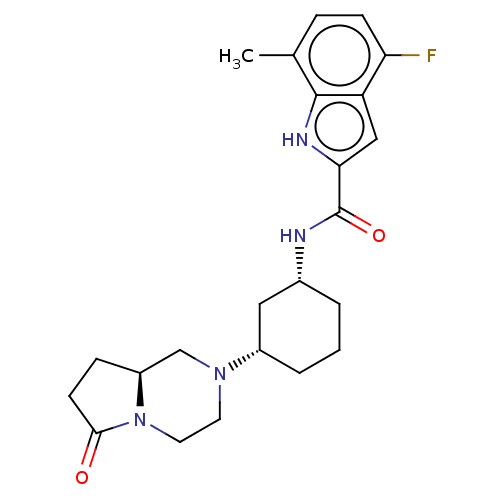
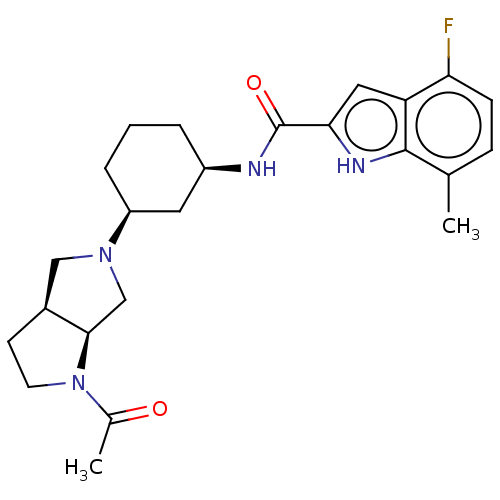
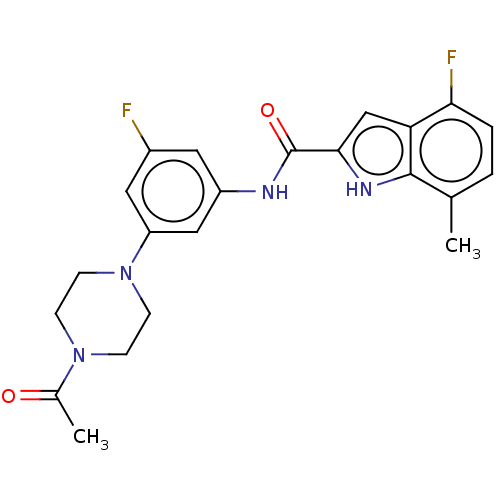
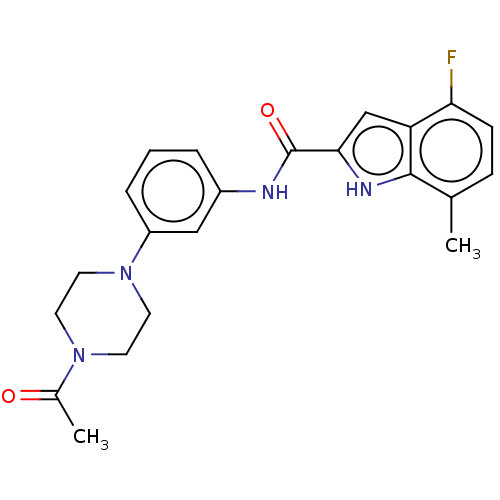
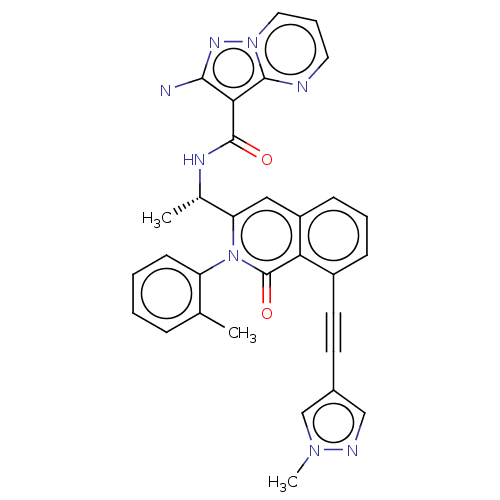
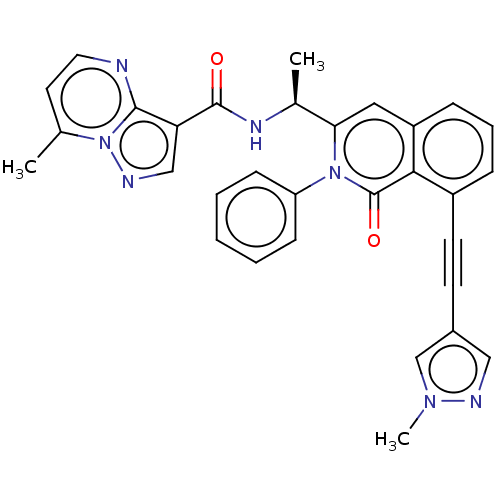
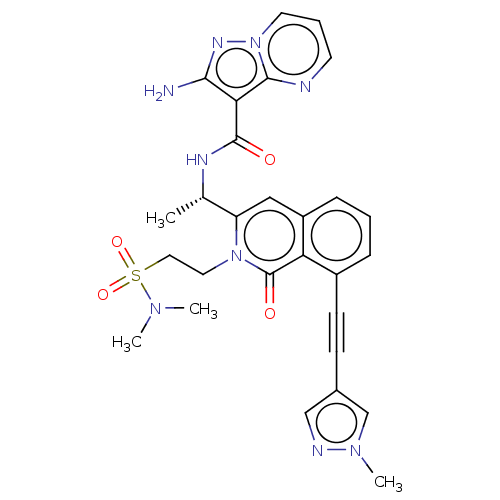
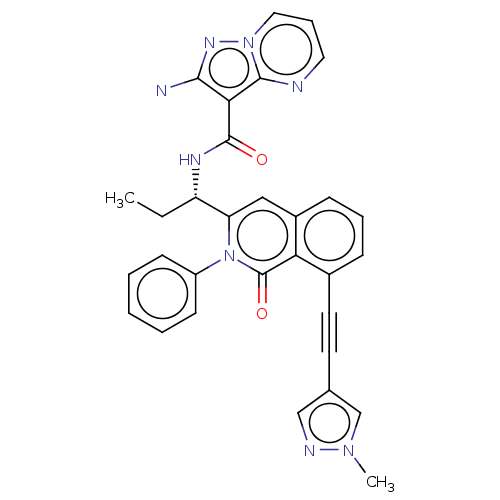
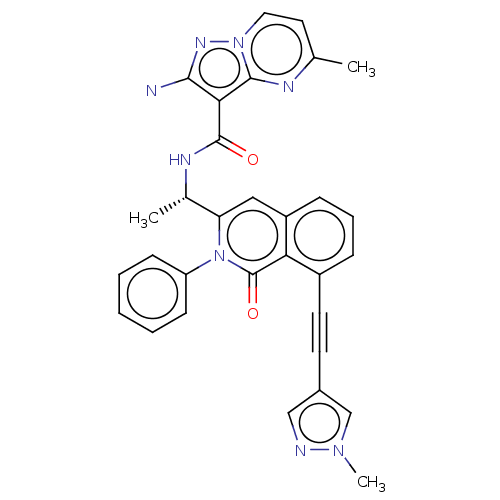
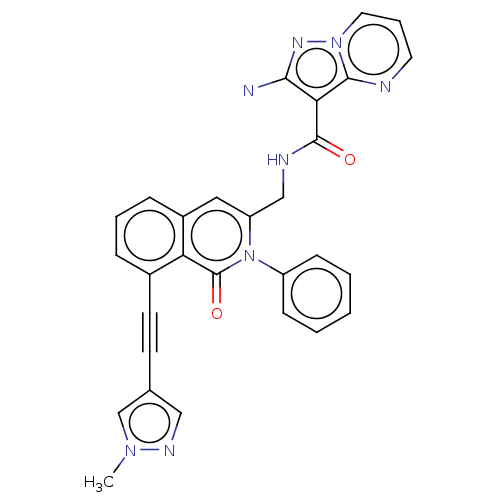
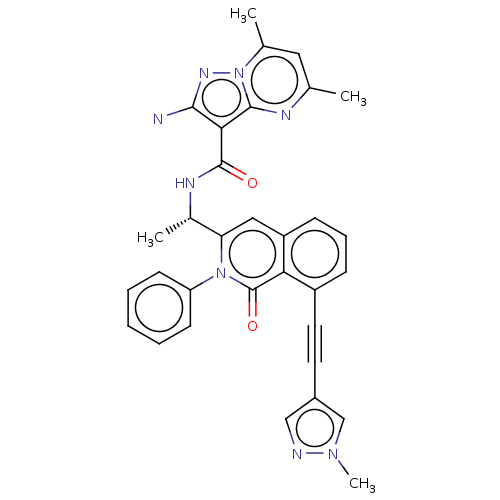
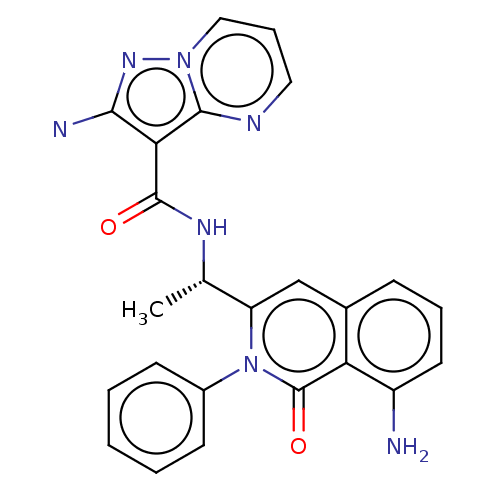
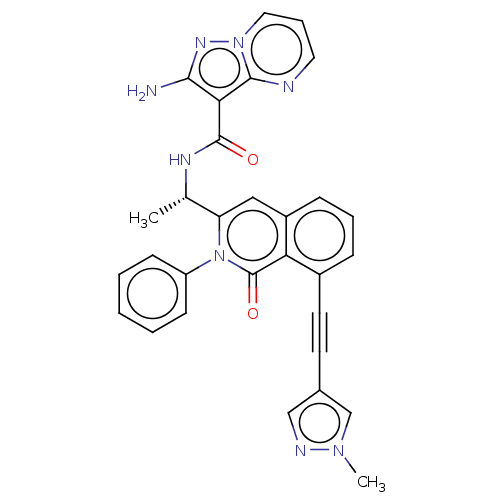
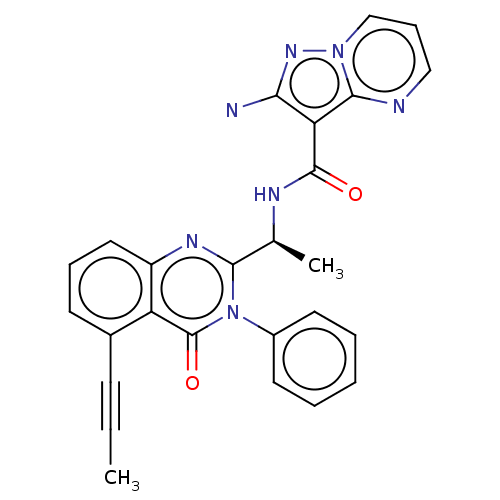
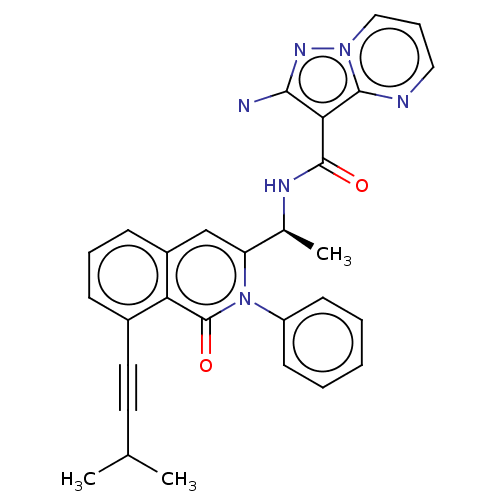
Affinity DataIC50: 5nMAssay Description:Inhibition of SETD2 in human A549 cells assessed as reduction in H3K36me3 incubated for 3 days by in-cell western assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of SETD2 (1434 to 1711 residues) (unknown origin) using SAM and biotin-Ahx-RKSAPATGGVKKPHR-NH2 as substrate preincubated for 30 mins follo...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of SETD2 (1434 to 1711 residues) (unknown origin) using SAM and biotin-Ahx-RKSAPATGGVKKPHR-NH2 as substrate preincubated for 30 mins follo...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of SETD2 in human A549 cells assessed as reduction in H3K36me3 incubated for 3 days by in-cell western assayMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of SETD2 (1434 to 1711 residues) (unknown origin) using SAM and biotin-Ahx-RKSAPATGGVKKPHR-NH2 as substrate preincubated for 30 mins follo...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of SETD2 (1434 to 1711 residues) (unknown origin) using SAM and biotin-Ahx-RKSAPATGGVKKPHR-NH2 as substrate preincubated for 30 mins follo...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of SETD2 in human A549 cells assessed as reduction in H3K36me3 incubated for 3 days by in-cell western assayMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of SETD2 (1434 to 1711 residues) (unknown origin) using SAM and biotin-Ahx-RKSAPATGGVKKPHR-NH2 as substrate preincubated for 30 mins follo...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibition of SETD2 (1434 to 1711 residues) (unknown origin) using SAM and biotin-Ahx-RKSAPATGGVKKPHR-NH2 as substrate preincubated for 30 mins follo...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of SETD2 in human A549 cells assessed as reduction in H3K36me3 incubated for 3 days by in-cell western assayMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibition of SETD2 (1434 to 1711 residues) (unknown origin) using SAM and biotin-Ahx-RKSAPATGGVKKPHR-NH2 as substrate preincubated for 30 mins follo...More data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:Inhibition of SETD2 in human A549 cells assessed as reduction in H3K36me3 incubated for 3 days by in-cell western assayMore data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Inhibition of SETD2 in human A549 cells assessed as reduction in H3K36me3 incubated for 3 days by in-cell western assayMore data for this Ligand-Target Pair
Affinity DataIC50: 134nMAssay Description:Inhibition of SETD2 in human A549 cells assessed as reduction in H3K36me3 incubated for 3 days by in-cell western assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.24E+3nMAssay Description:Inhibition of SETD2 (1434 to 1711 residues) (unknown origin) using SAM and biotin-Ahx-RKSAPATGGVKKPHR-NH2 as substrate preincubated for 30 mins follo...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 2.00E+3nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair

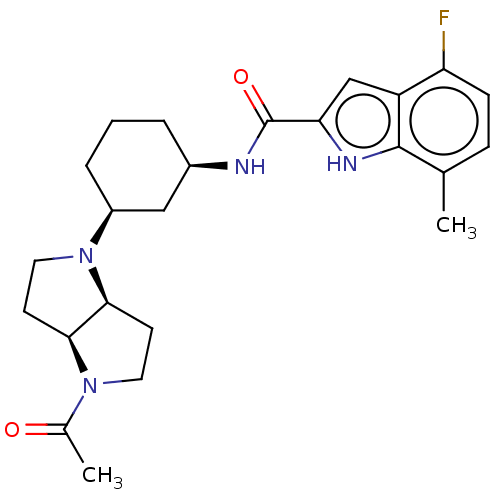
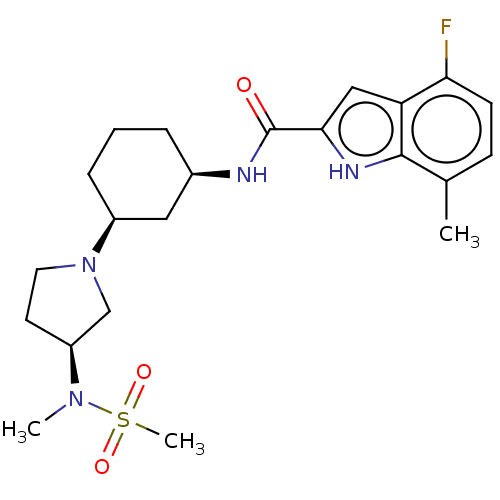
 3D Structure (crystal)
3D Structure (crystal)