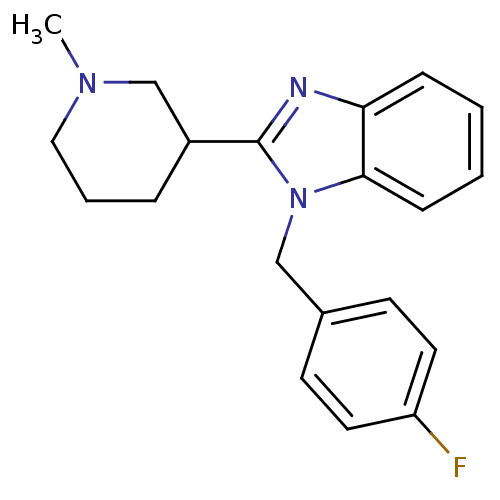
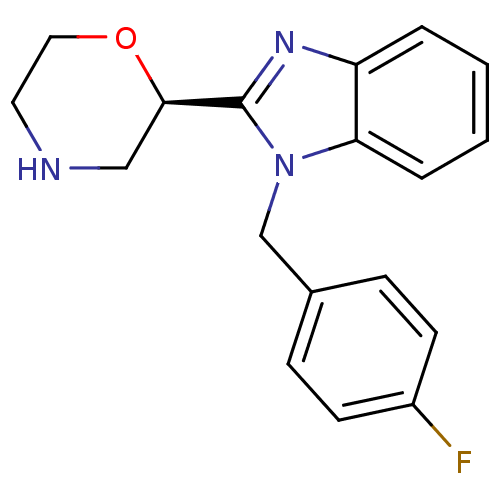
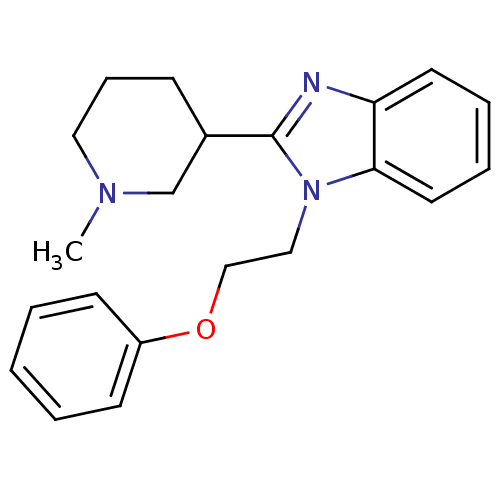
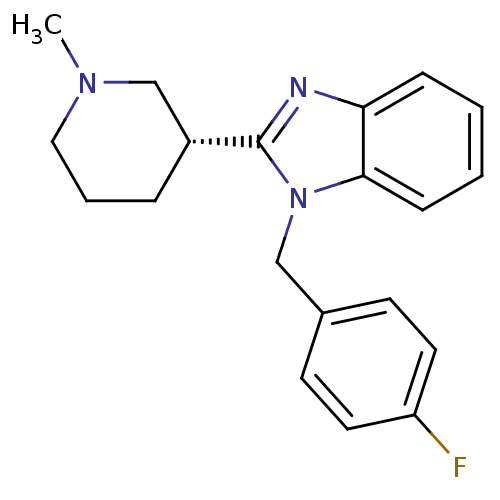
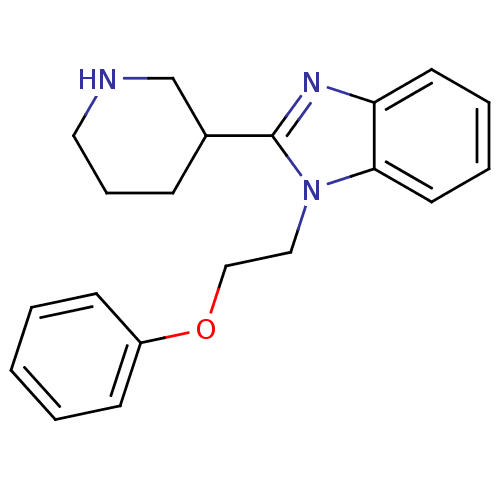
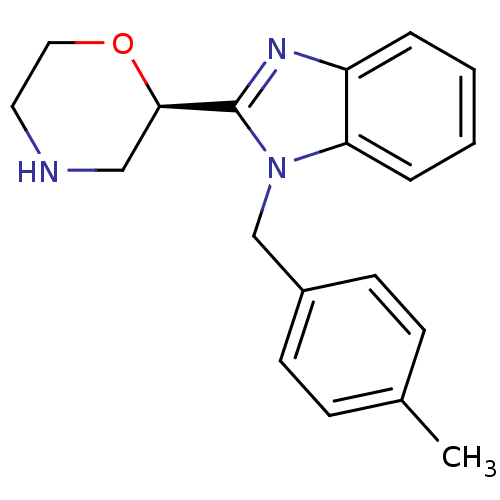
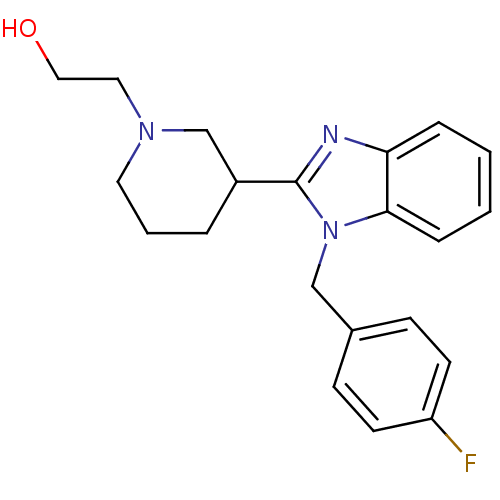
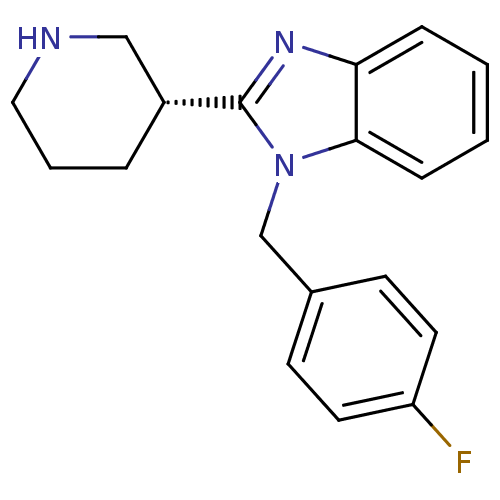
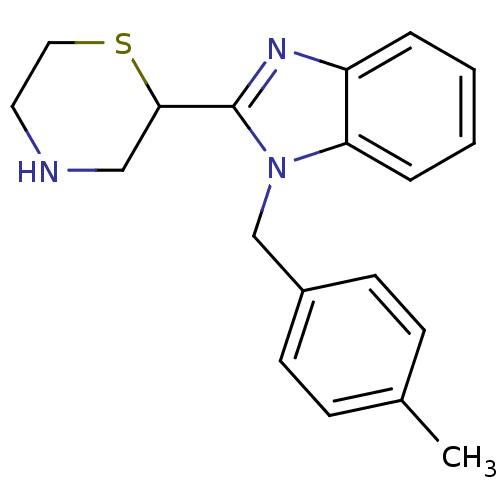
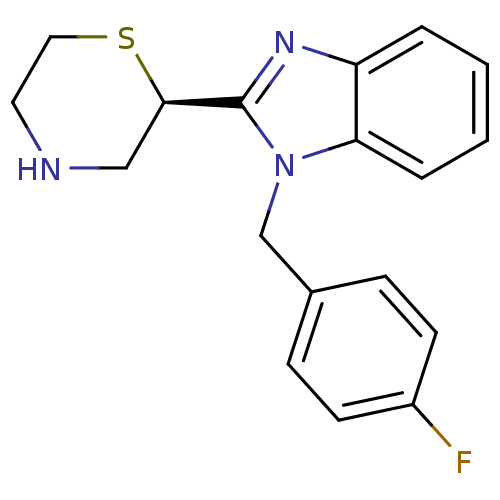
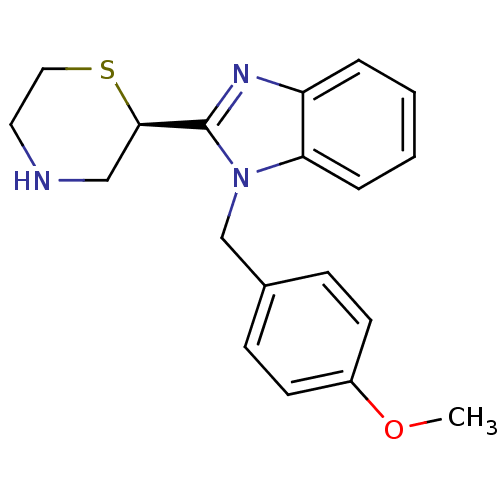
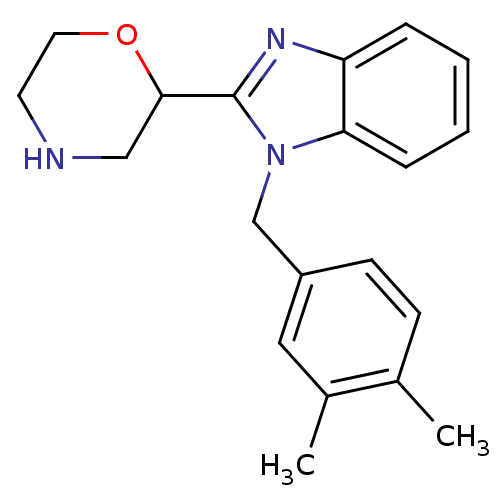
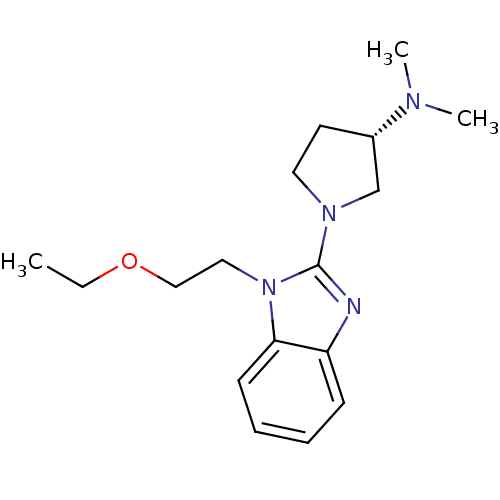
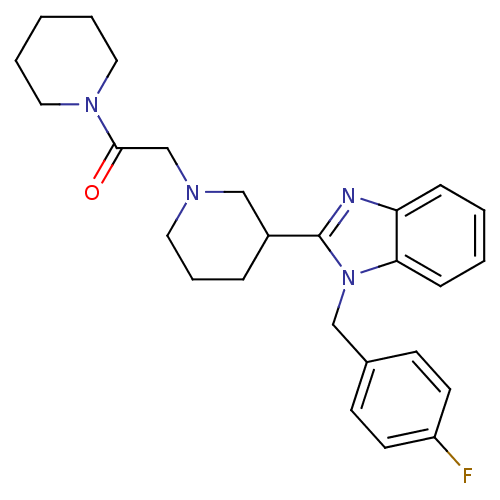
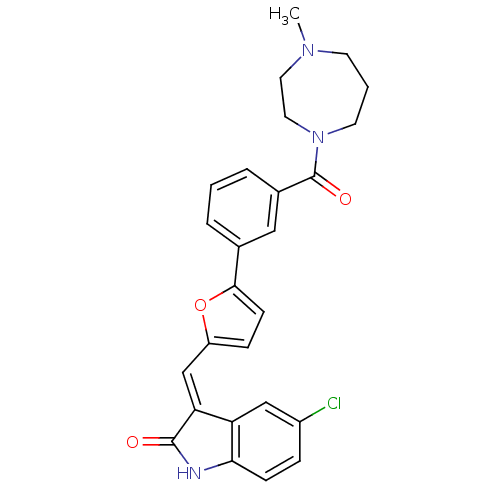
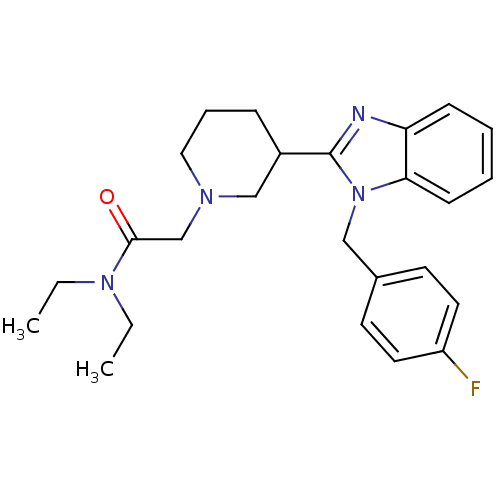
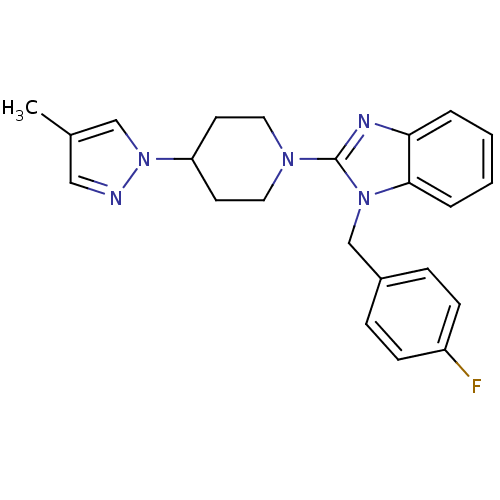
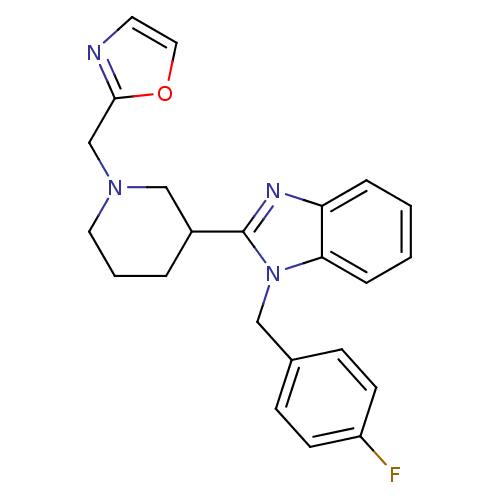
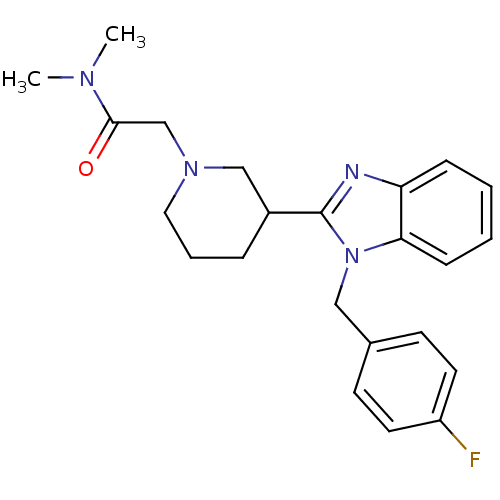
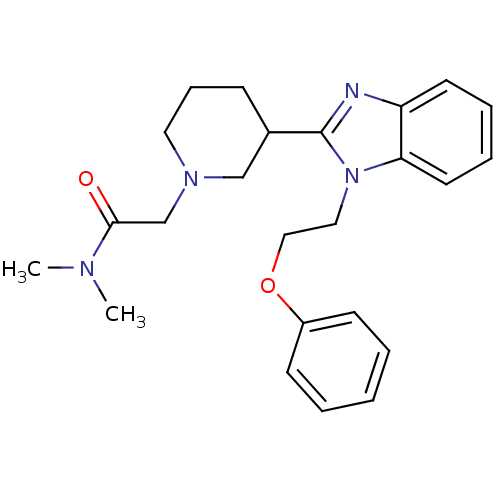
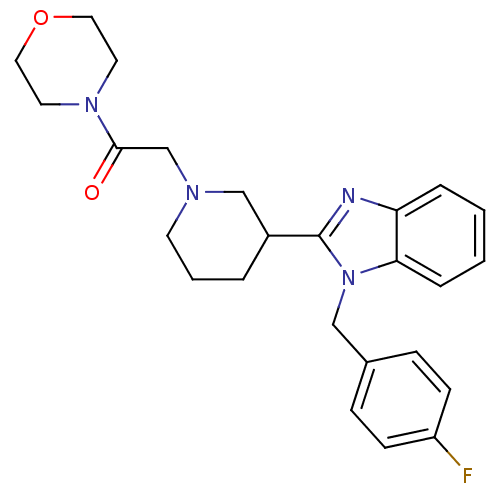
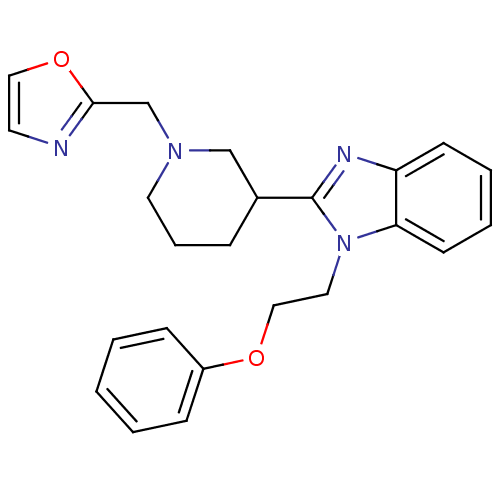
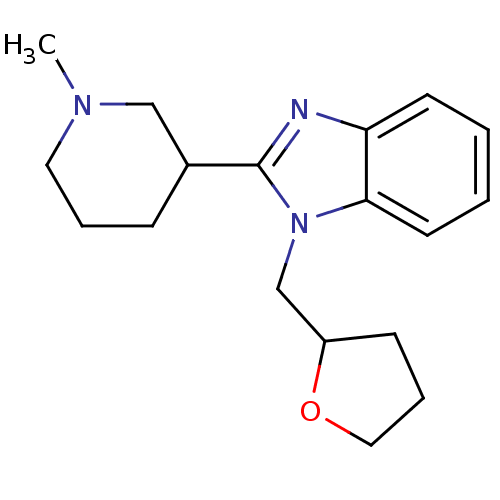
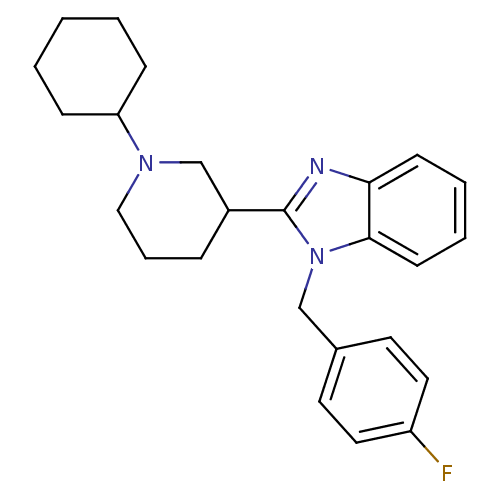
Affinity DataKi: 0.5nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.90nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.90nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.90nMAssay Description:Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 3.90nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4.80nMAssay Description:Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 4.90nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Reversible inhibition of human recombinant Pim1 using RSRHSSYPAGT as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.90nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.90nMAssay Description:Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 8.10nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 8.10nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 8.40nMAssay Description:Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 8.90nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 8.90nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 11.9nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 11.9nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 16.6nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 24.6nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Inhibition of muscarinic M1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 92nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 230nMAssay Description:Inhibition of muscarinic M1 receptorMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 340nMAssay Description:Inhibition of muscarinic M1 receptorMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 430nMAssay Description:Inhibition of muscarinic M1 receptorMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 480nMAssay Description:Inhibition of muscarinic M1 receptorMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 638nMAssay Description:Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells by liquid scintillation countingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 926nMAssay Description:Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells by liquid scintillation countingMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 930nMAssay Description:Inhibition of muscarinic M1 receptorMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 1.18E+3nMAssay Description:Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells by liquid scintillation countingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 1.26E+3nMAssay Description:Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells by liquid scintillation countingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 1.37E+3nMAssay Description:Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells by liquid scintillation countingMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 1.50E+3nMAssay Description:Displacement of [3H]N-methyl scopolamine from human muscarinic M1 receptor expressed in CHO Flp-In cells by liquid scintillation countingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 1.84E+3nMAssay Description:Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells by liquid scintillation countingMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 2.50E+3nMAssay Description:Displacement of [3H]N-methyl scopolamine from human muscarinic M1 receptor expressed in CHO Flp-In cells by liquid scintillation countingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 2.60E+3nMAssay Description:Displacement of [3H]dofetolide from human ErgMore data for this Ligand-Target Pair