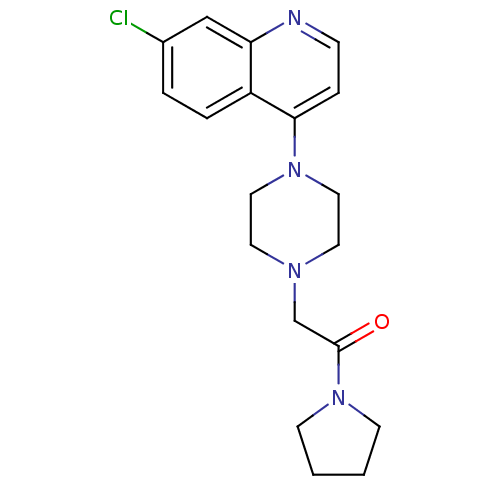
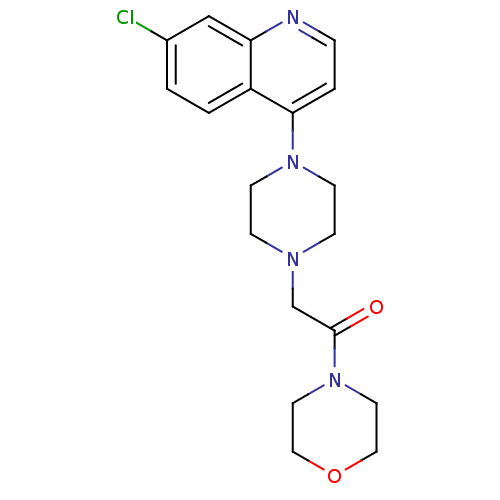
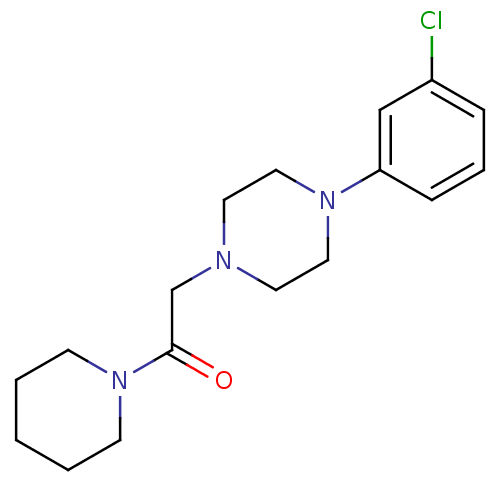
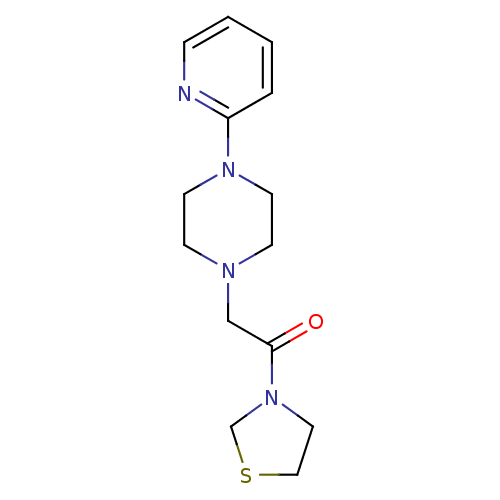
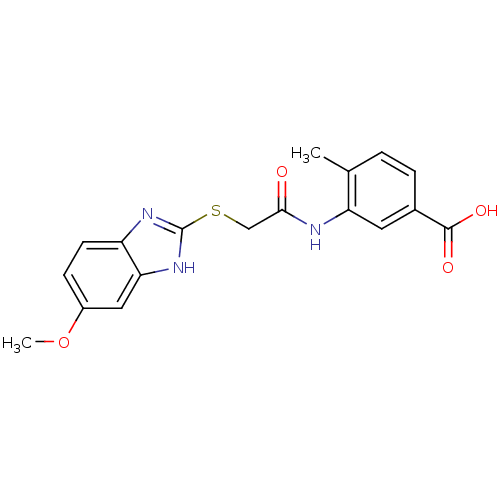
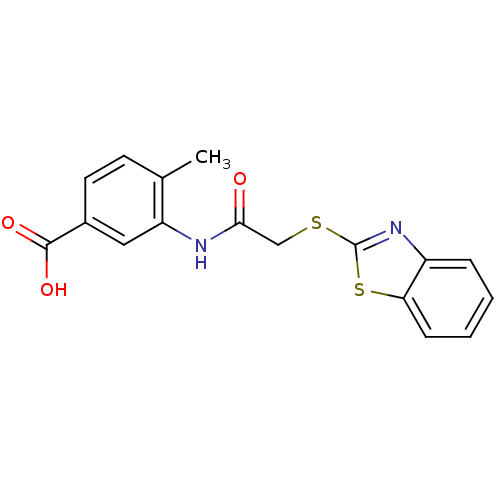
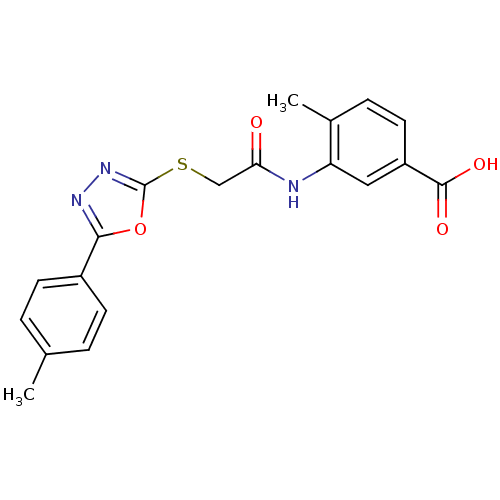
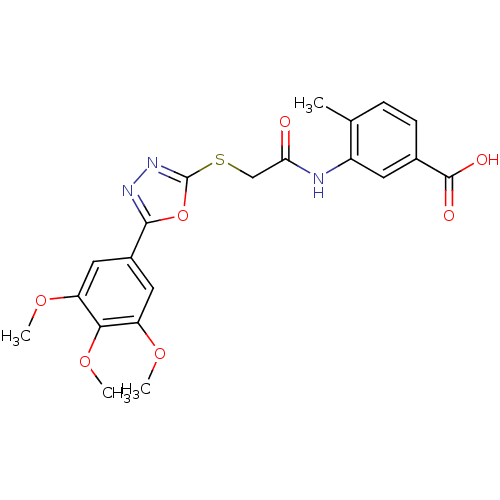
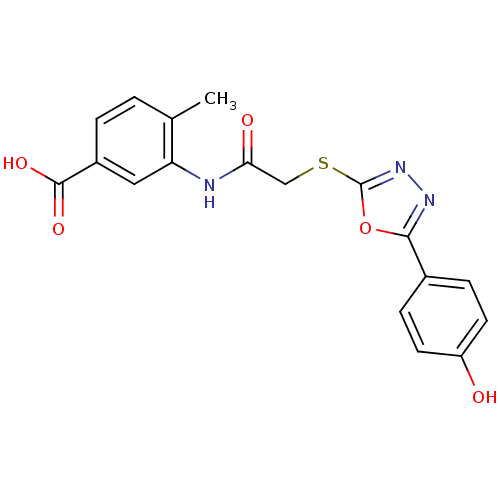
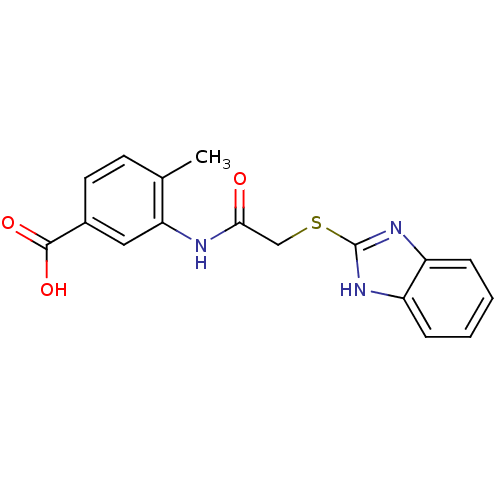
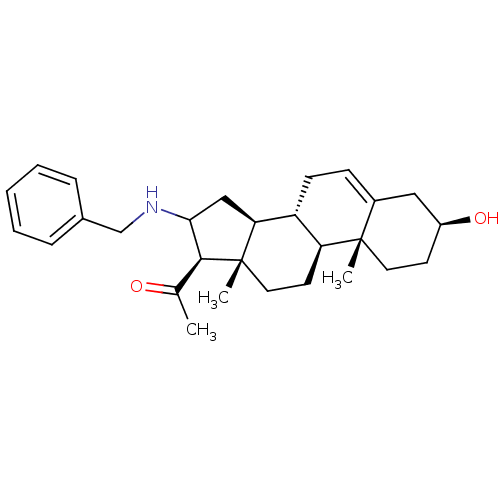
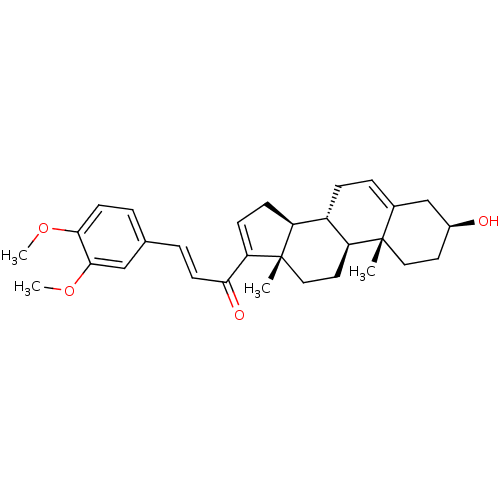
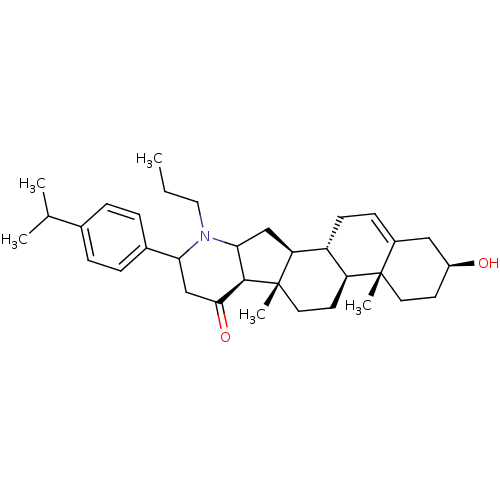
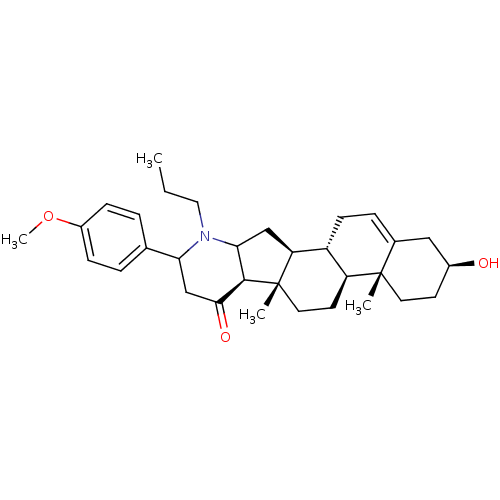
Affinity DataIC50: 18nMAssay Description:DPP-IV inhibitory activity was determined by measuring the p-nitroaniline (pNA) released from the chromogenic substrate hydrolysis (H-Gly-Pro-pNA). T...More data for this Ligand-Target Pair
Affinity DataIC50: 3.73E+3nMAssay Description:DPP-IV inhibitory activity was determined by measuring the p-nitroaniline (pNA) released from the chromogenic substrate hydrolysis (H-Gly-Pro-pNA). T...More data for this Ligand-Target Pair
Affinity DataIC50: 5.96E+3nMAssay Description:DPP-IV inhibitory activity was determined by measuring the p-nitroaniline (pNA) released from the chromogenic substrate hydrolysis (H-Gly-Pro-pNA). T...More data for this Ligand-Target Pair
Affinity DataIC50: 6.06E+3nMAssay Description:DPP-IV inhibitory activity was determined by measuring the p-nitroaniline (pNA) released from the chromogenic substrate hydrolysis (H-Gly-Pro-pNA). T...More data for this Ligand-Target Pair
Affinity DataIC50: 6.08E+3nMAssay Description:DPP-IV inhibitory activity was determined by measuring the p-nitroaniline (pNA) released from the chromogenic substrate hydrolysis (H-Gly-Pro-pNA). T...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
School Of Pharmaceutical Sciences
Curated by ChEMBL
School Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 8.20E+3nMAssay Description:Inhibition of recombinant human PTP1B by malachite green assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
School Of Pharmaceutical Sciences
Curated by ChEMBL
School Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 8.50E+3nMAssay Description:Inhibition of recombinant human PTP1B by malachite green assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
School Of Pharmaceutical Sciences
Curated by ChEMBL
School Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 8.90E+3nMAssay Description:Inhibition of recombinant human PTP1B by malachite green assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
School Of Pharmaceutical Sciences
Curated by ChEMBL
School Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 9.70E+3nMAssay Description:Inhibition of recombinant human PTP1B by malachite green assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
School Of Pharmaceutical Sciences
Curated by ChEMBL
School Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 1.03E+4nMAssay Description:Inhibition of recombinant human PTP1B by malachite green assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
School Of Pharmaceutical Sciences
Curated by ChEMBL
School Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 1.52E+4nMAssay Description:Inhibition of recombinant human PTP1B by malachite green assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.13E+4nMAssay Description:Inhibition of human DPP-4 assessed as cleavage of substrate using H-Gly-Pro-AMC chromogenic substrate after 10 mins by double beam spectrophotometerMore data for this Ligand-Target Pair
Affinity DataIC50: 2.62E+4nMAssay Description:Inhibition of human DPP-4 assessed as cleavage of substrate using H-Gly-Pro-AMC chromogenic substrate after 10 mins by double beam spectrophotometerMore data for this Ligand-Target Pair
Affinity DataIC50: 2.91E+4nMAssay Description:Inhibition of human DPP-4 assessed as cleavage of substrate using H-Gly-Pro-AMC chromogenic substrate after 10 mins by double beam spectrophotometerMore data for this Ligand-Target Pair
Affinity DataIC50: 3.27E+4nMAssay Description:Inhibition of human DPP-4 assessed as cleavage of substrate using H-Gly-Pro-AMC chromogenic substrate after 10 mins by double beam spectrophotometerMore data for this Ligand-Target Pair
Affinity DataIC50: 3.56E+4nMAssay Description:Inhibition of human DPP-4 assessed as cleavage of substrate using H-Gly-Pro-AMC chromogenic substrate after 10 mins by double beam spectrophotometerMore data for this Ligand-Target Pair

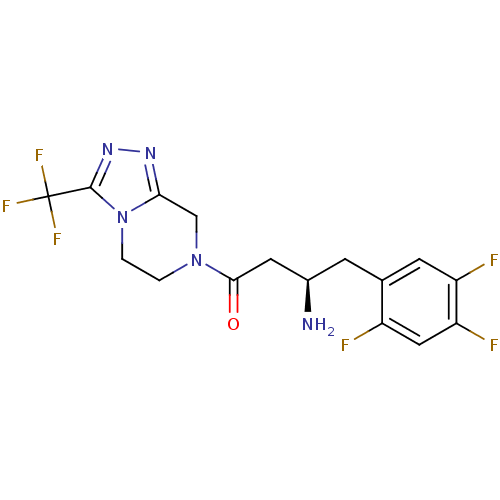
 3D Structure (crystal)
3D Structure (crystal)