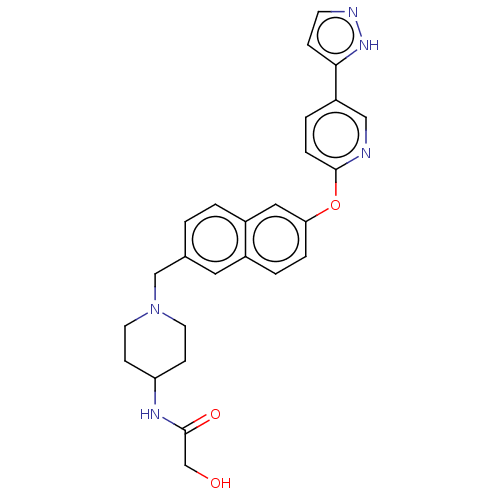
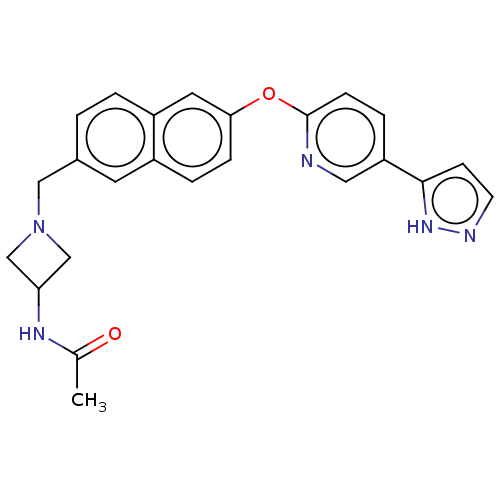
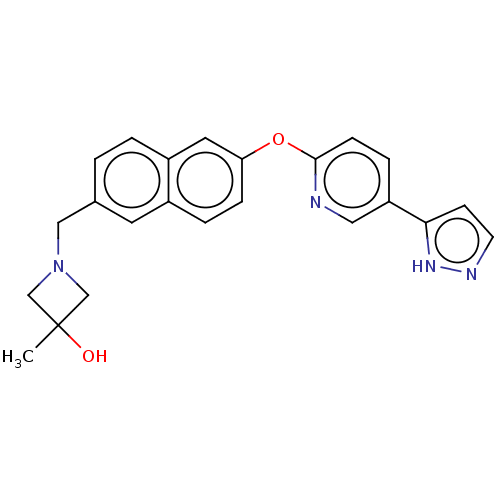
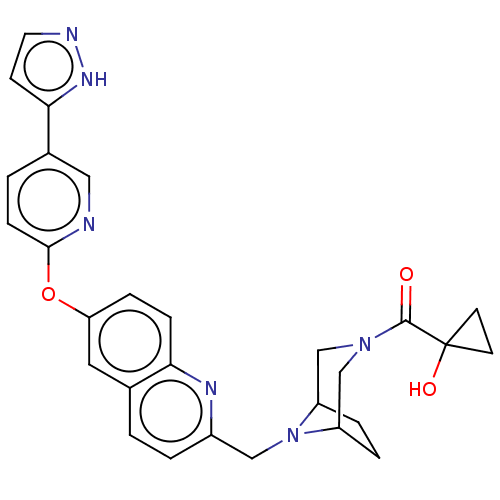
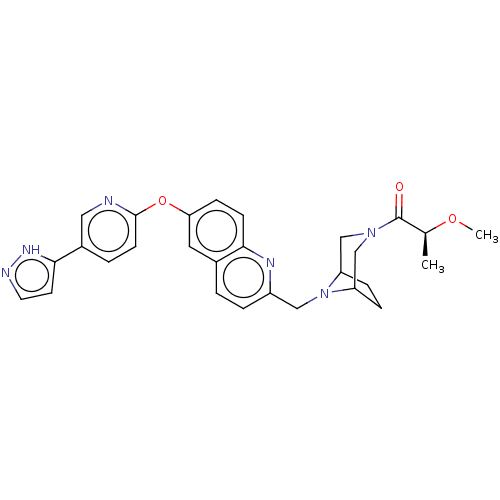
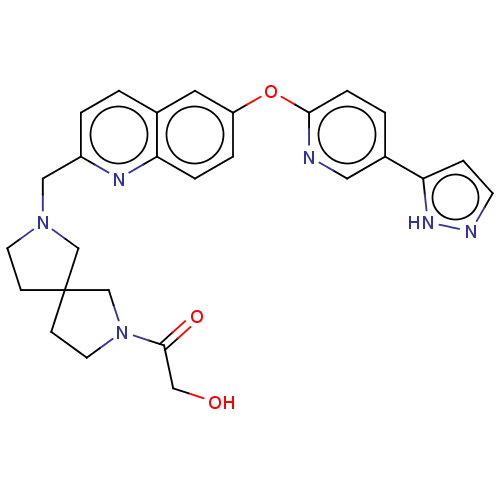
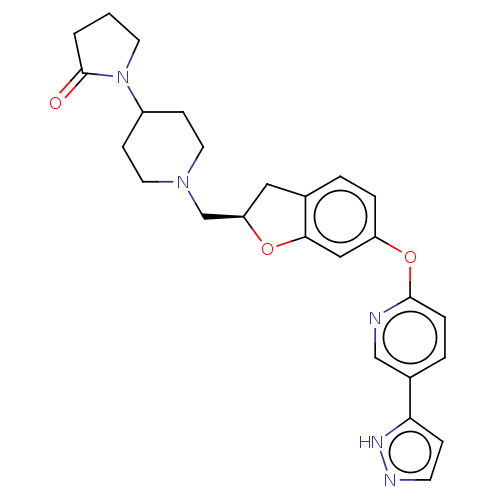
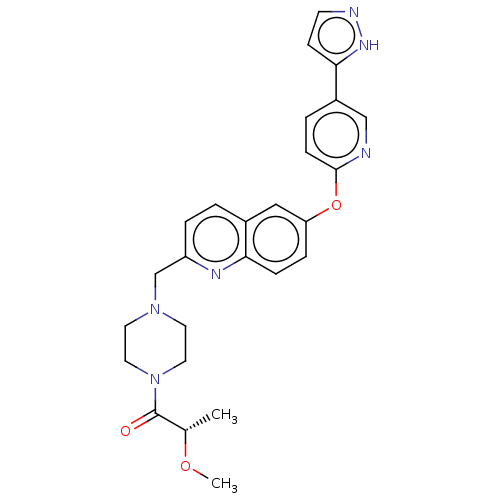
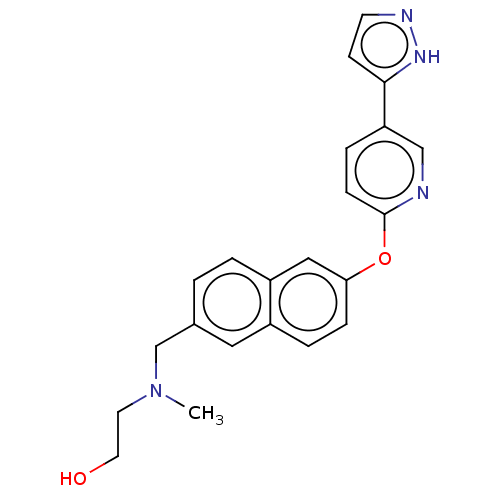
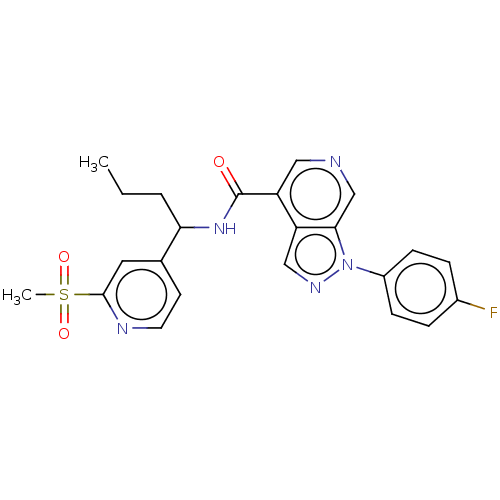
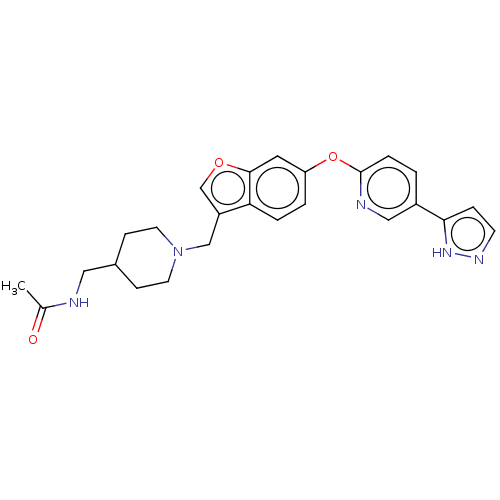
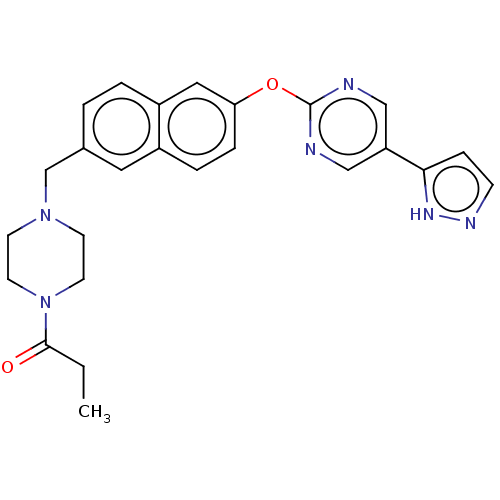
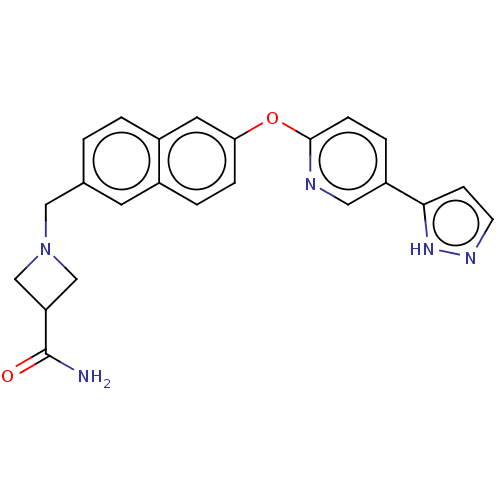
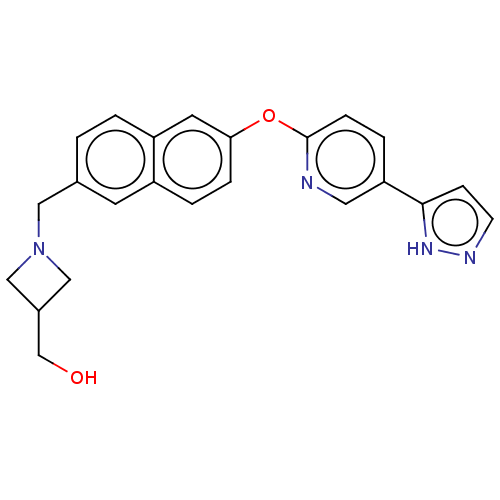
TargetC-C chemokine receptor type 1(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
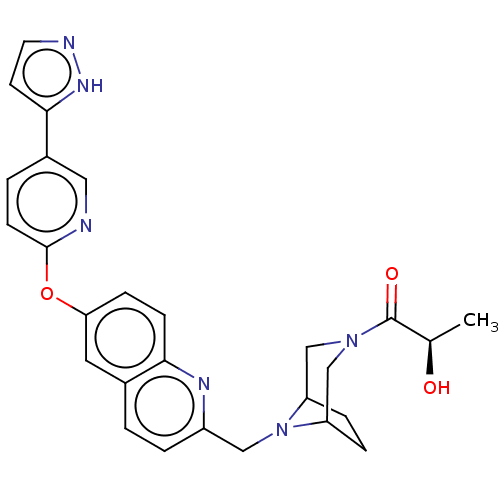
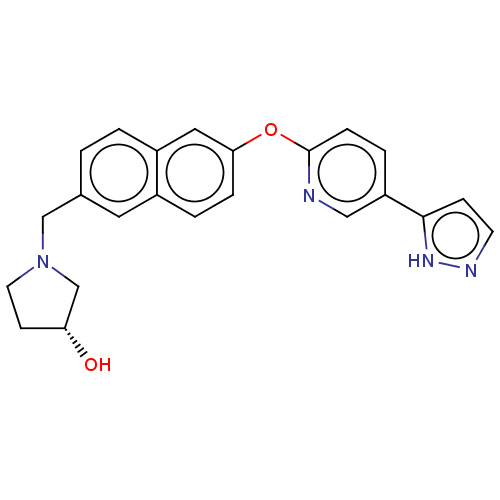
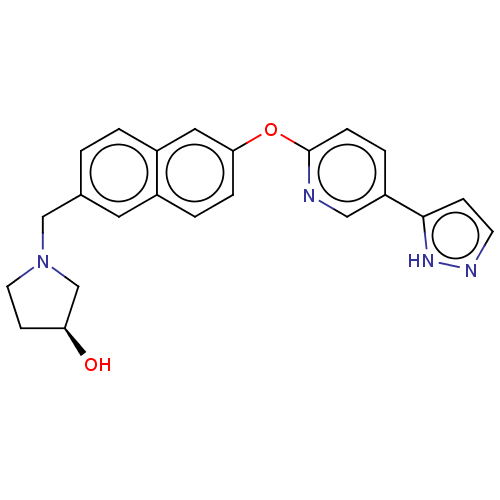
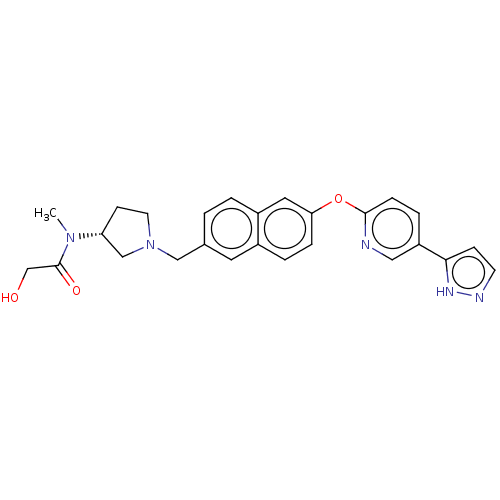
Affinity DataIC50: 0.200nMAssay Description:Antagonist activity at recombinant CCR1 (unknown origin) expressed in non-adherent cells co-expressing Galpha16 assessed as inhibition of MIP-1 alpha...More data for this Ligand-Target Pair
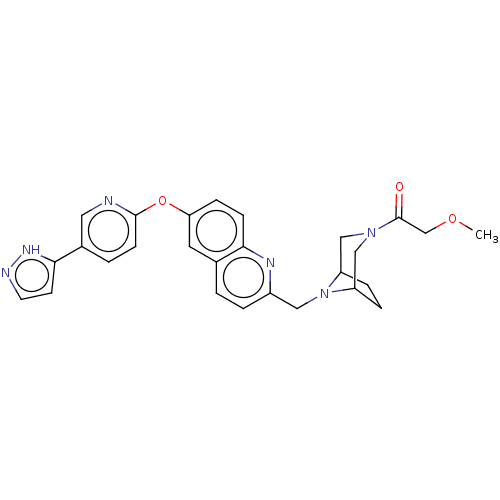
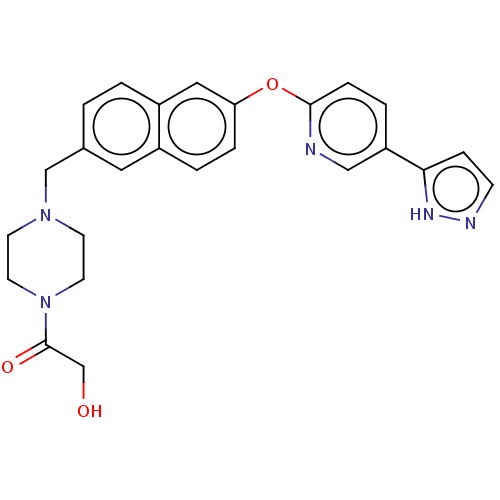
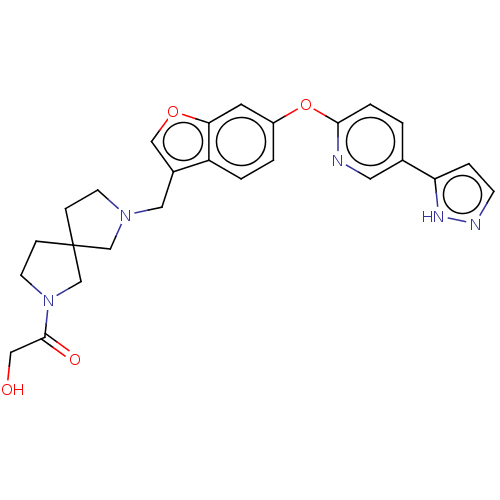
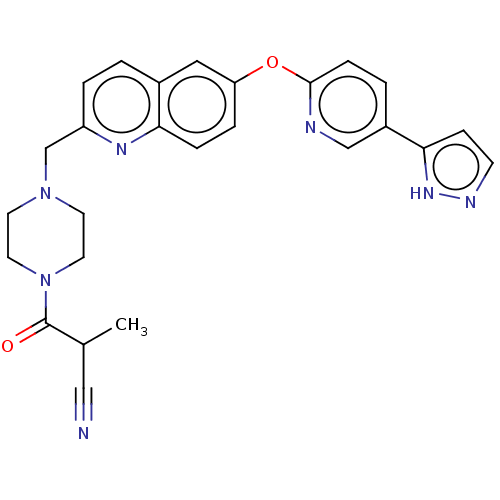
TargetC-C chemokine receptor type 1(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
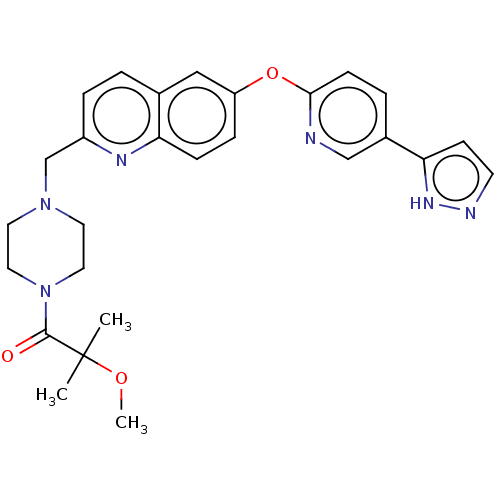
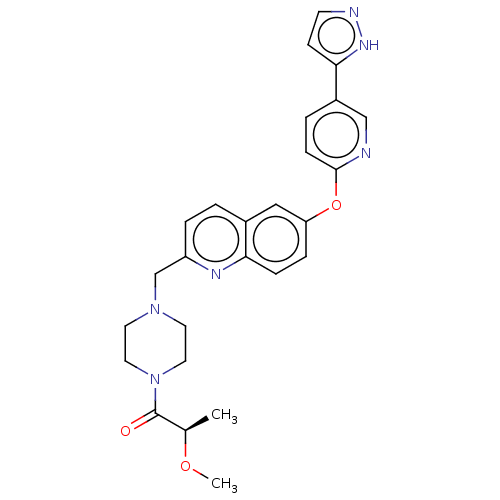
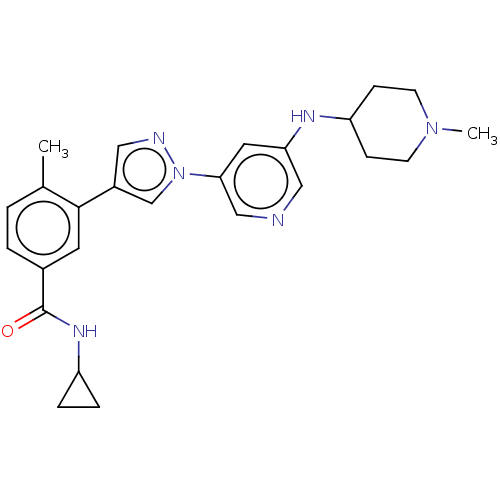
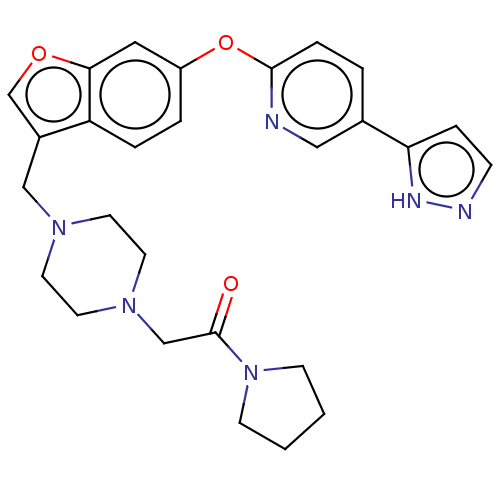
Affinity DataIC50: 0.200nMAssay Description:Antagonist activity at recombinant CCR1 (unknown origin) expressed in non-adherent cells co-expressing Galpha16 assessed as inhibition of MIP-1 alpha...More data for this Ligand-Target Pair
Affinity DataIC50: 0.220nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
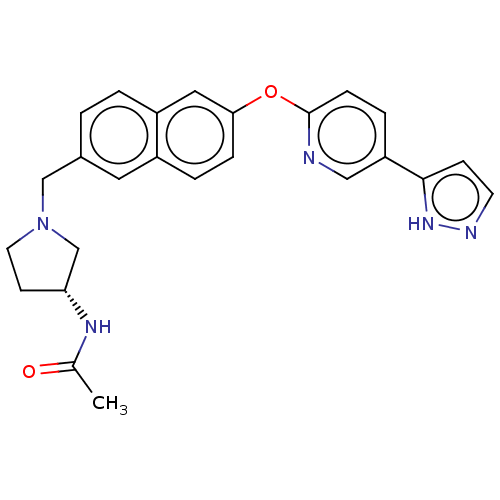
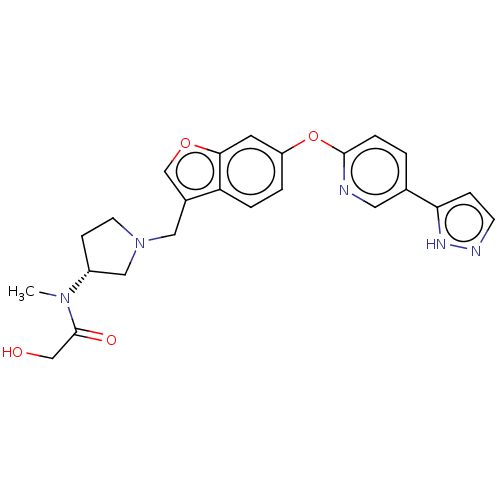
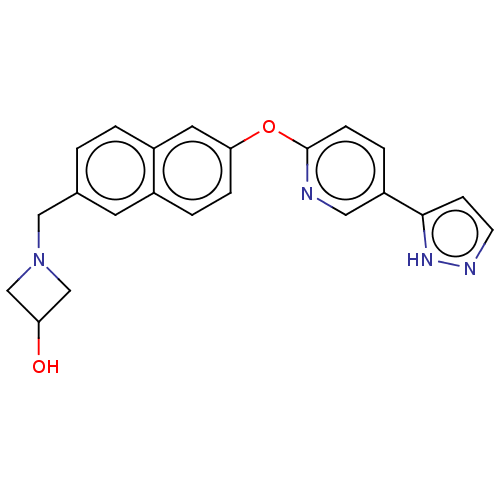
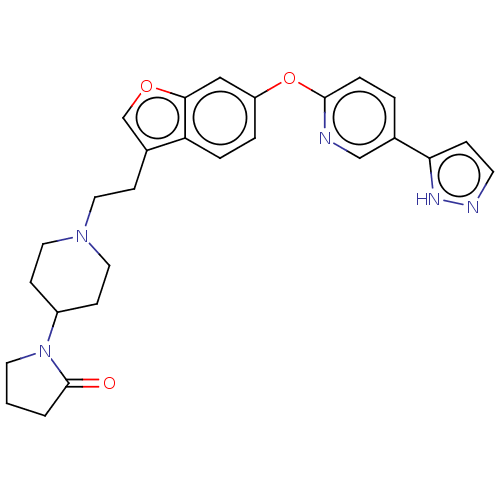
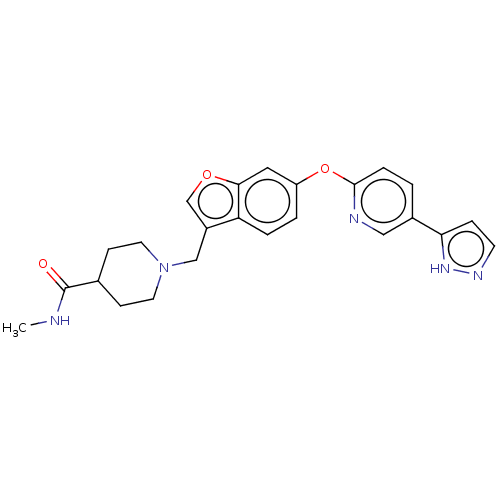
TargetC-C chemokine receptor type 1(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
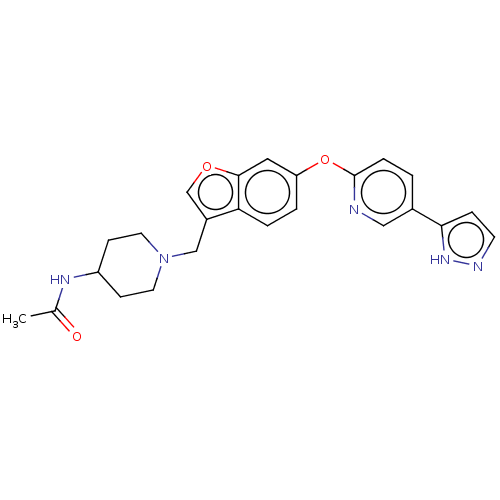
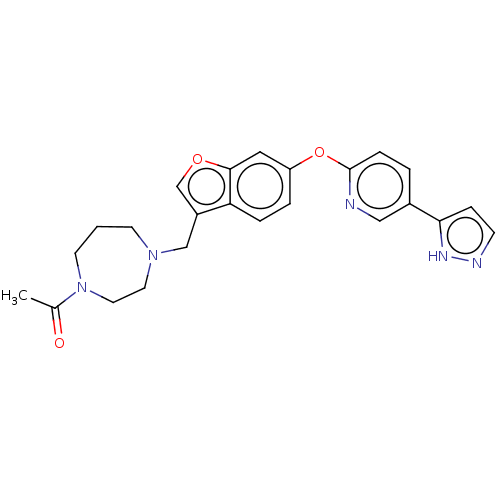
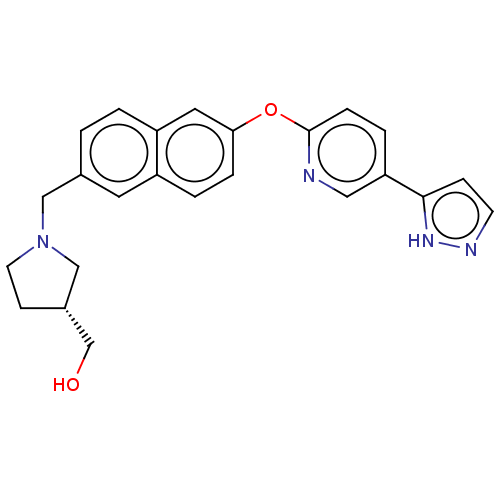
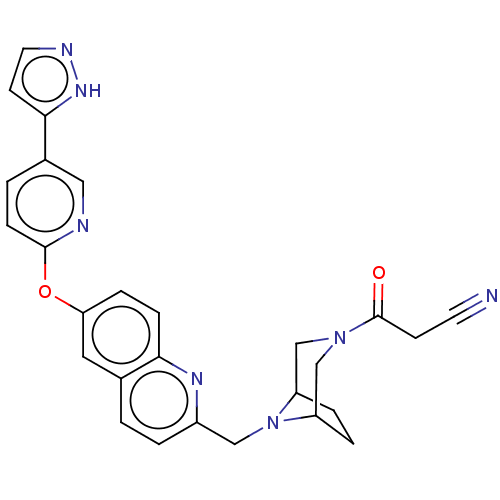
Affinity DataIC50: 0.300nMAssay Description:Antagonist activity at recombinant CCR1 (unknown origin) expressed in non-adherent cells co-expressing Galpha16 assessed as inhibition of MIP-1 alpha...More data for this Ligand-Target Pair
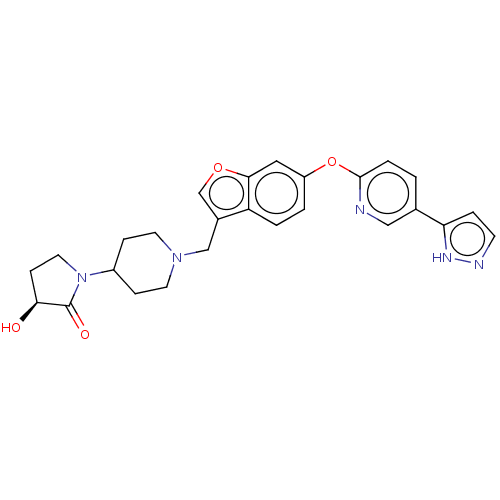
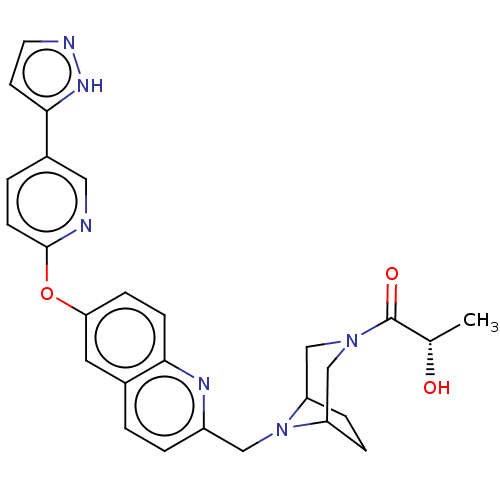
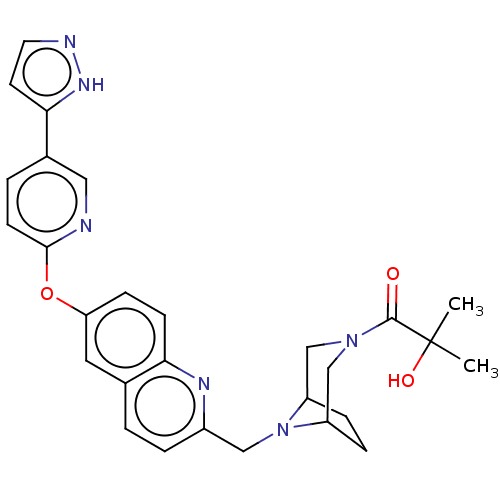
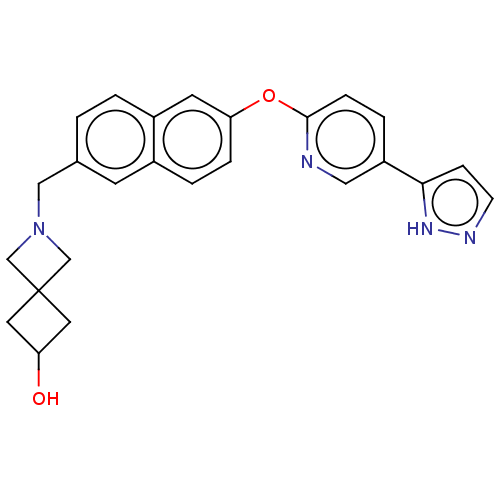
TargetC-C chemokine receptor type 1(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
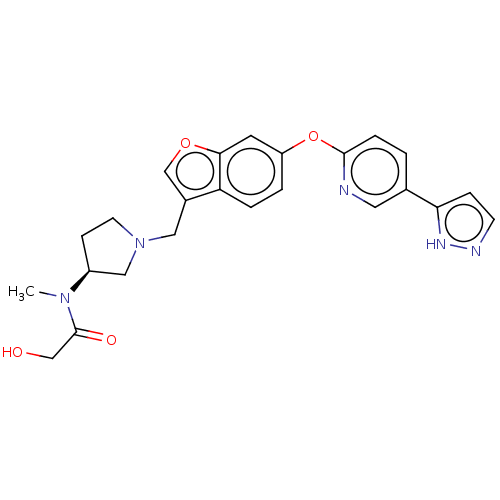
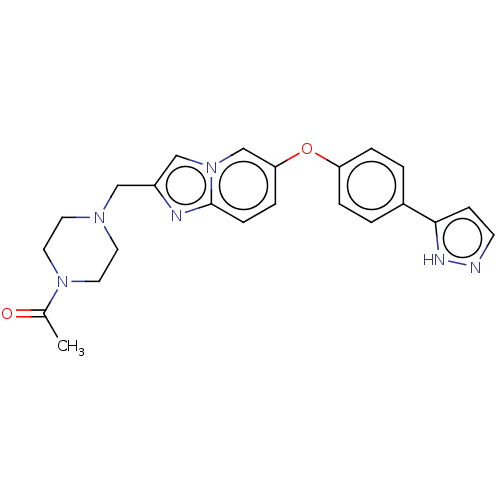
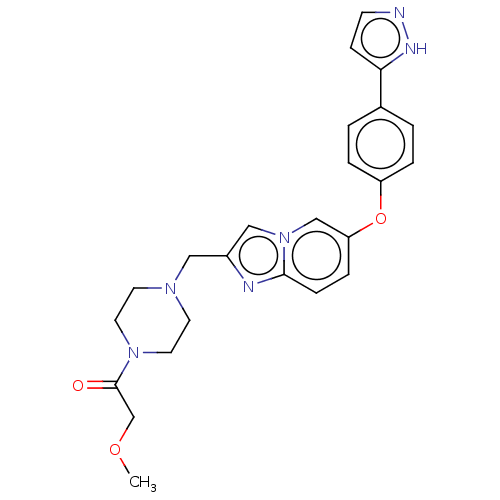
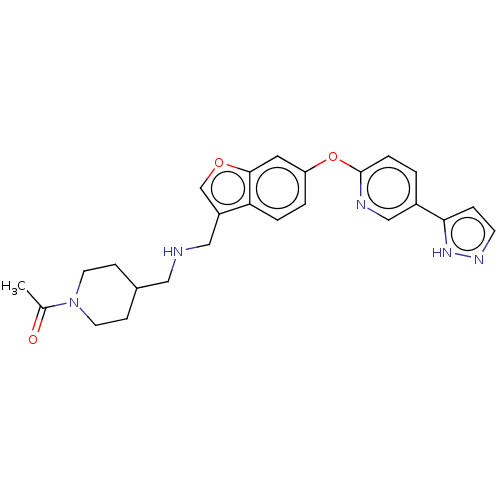
Affinity DataIC50: 0.300nMAssay Description:Antagonist activity at CCR1 in human THP1 cells assessed as inhibition of chemotaxis after 30 mins by Celltiter-glo reagent based luminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.330nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.340nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.360nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.380nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.420nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.450nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.460nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.470nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.470nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.480nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.480nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.490nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
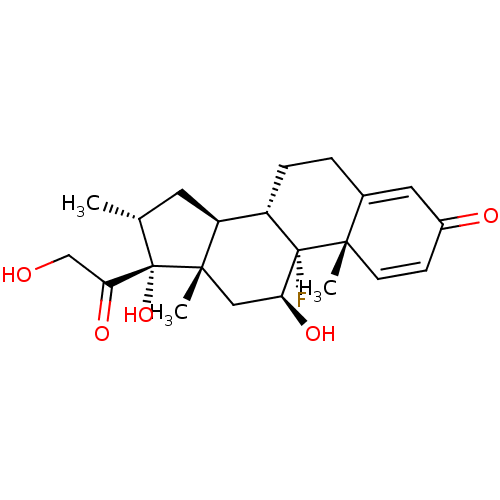
TargetGlucocorticoid receptor(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Agonist activity at glucocorticoid receptor in human foreskin fibroblasts assessed as inhibition of IL-1-induced IL-6 production by trans-repression ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.510nMAssay Description:Transrepression activity at glucocorticoid receptor in HFF assessed as inhibition of IL-1-induced IL-6 production after 18 to 24 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.530nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.530nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.540nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.560nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.560nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.560nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.570nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.570nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.580nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.590nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
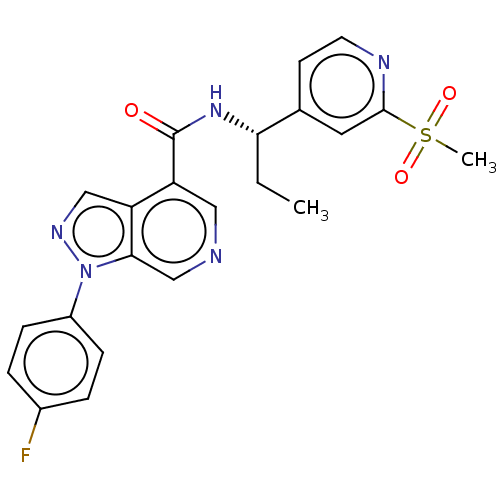
TargetReceptor-interacting serine/threonine-protein kinase 2(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 0.610nMAssay Description:In a 384-well plate, test compound diluted in assay buffer (1% DMSO final) is mixed with 8His-RIPK2 FL enzyme (final concentration of 8 nM). After 15...More data for this Ligand-Target Pair
Affinity DataIC50: 0.630nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.640nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.660nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.660nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.660nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.660nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.670nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.670nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.680nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.690nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMpH: 7.5 T: 2°CAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair

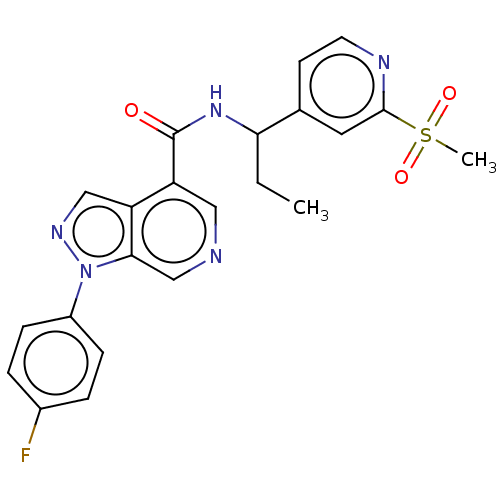
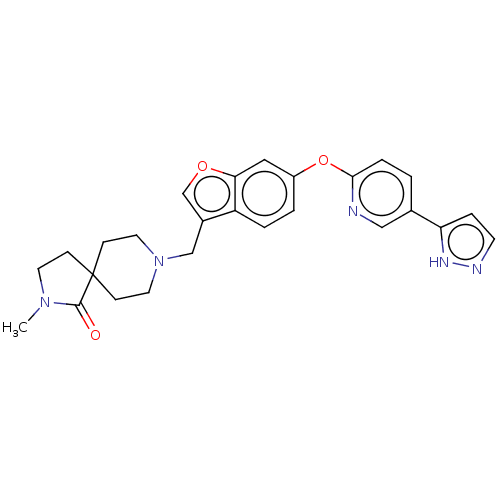
 3D Structure (crystal)
3D Structure (crystal)