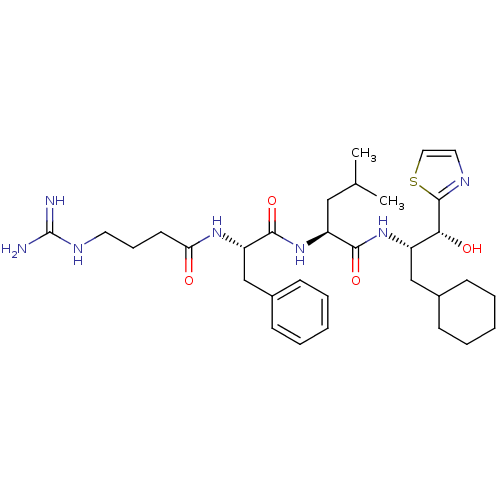
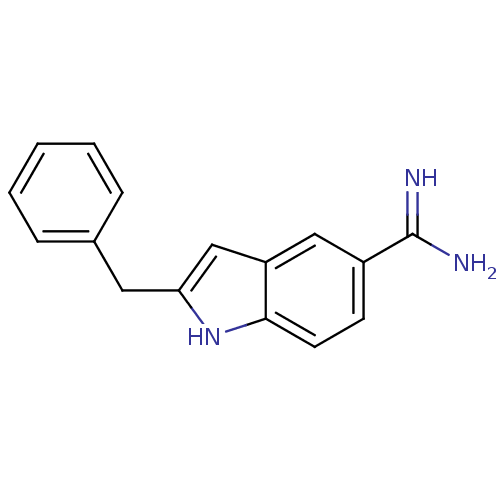
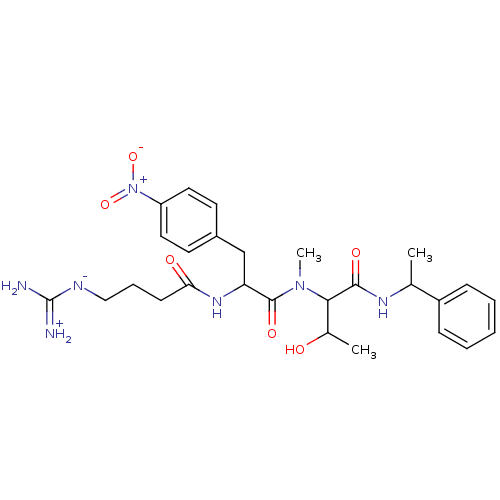
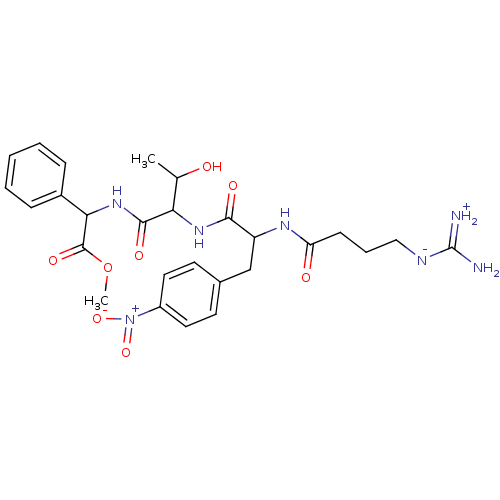
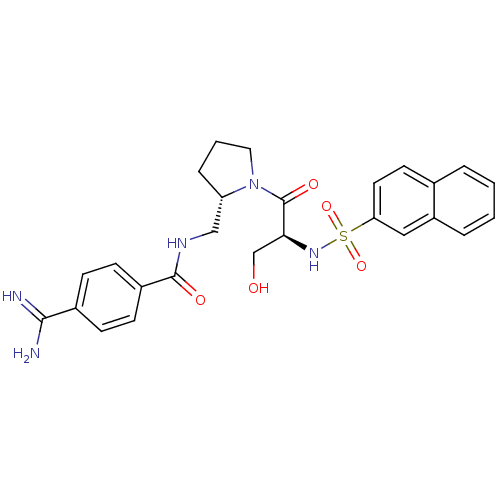
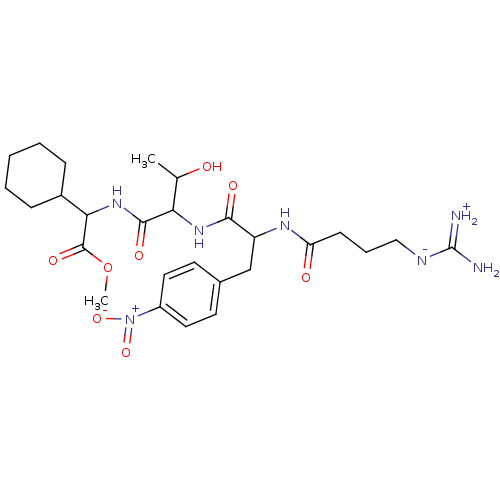
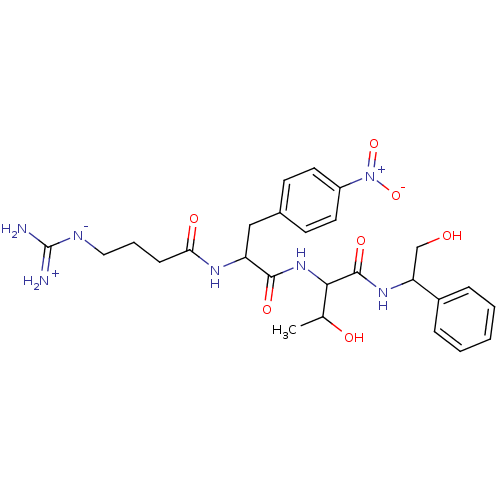
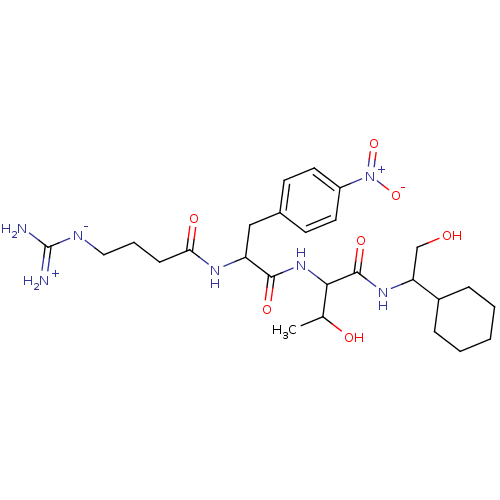
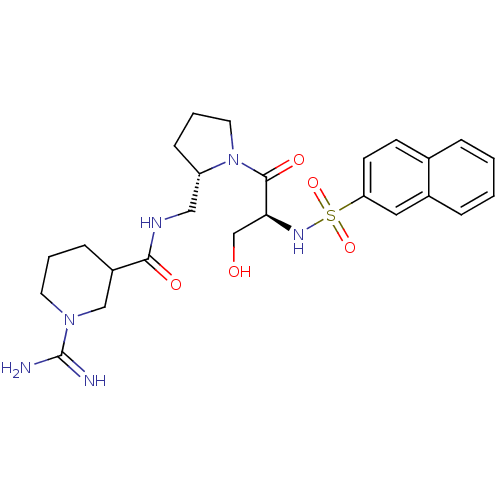
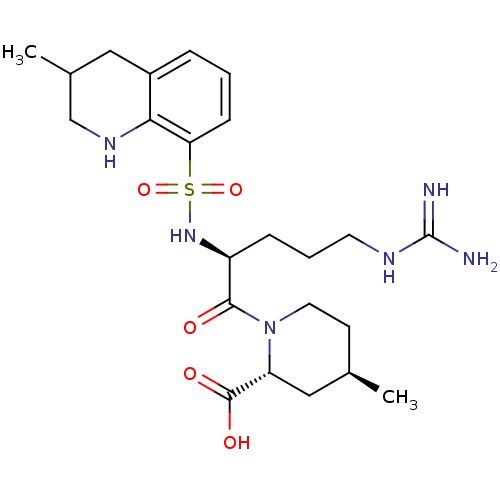
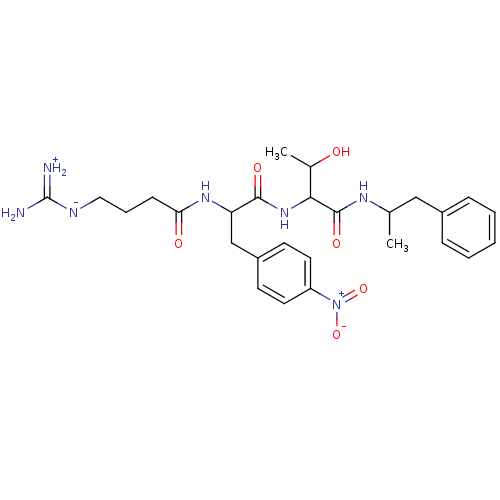
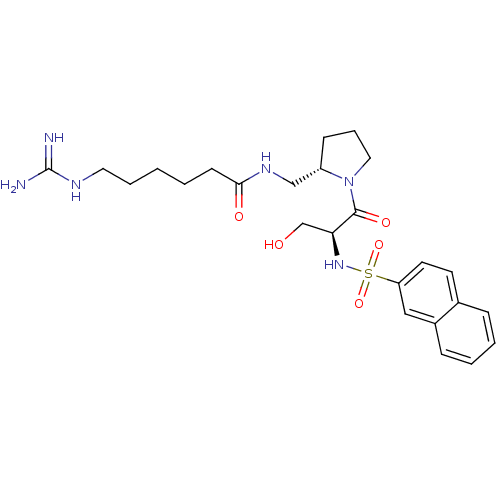
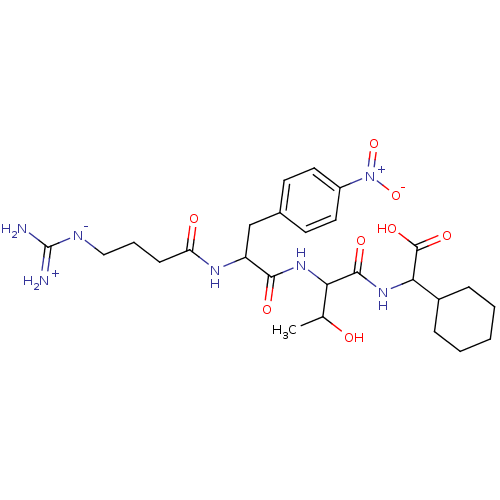
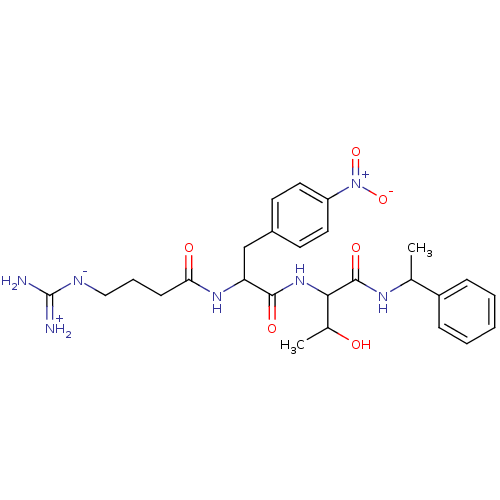
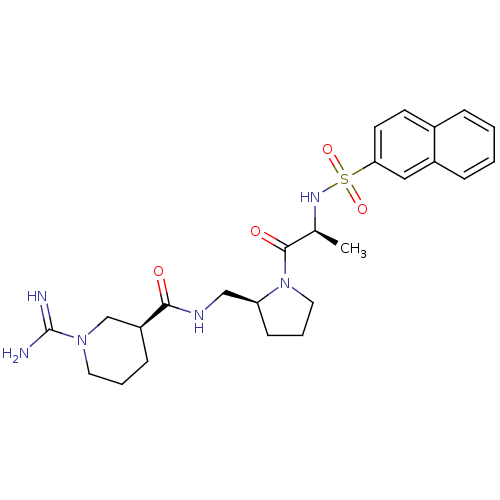
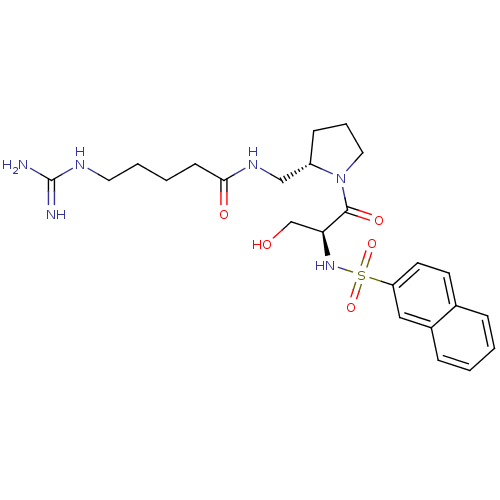
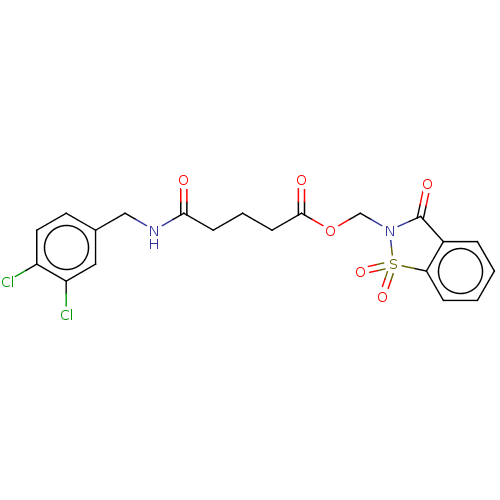
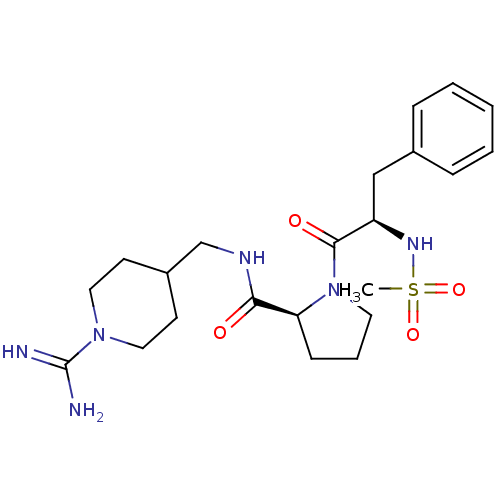
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.460nMAssay Description:Competitive kinetic for thrombin inhibition Ki was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Overall Inhibitory constant of the compound against thrombin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 3.40nMAssay Description:In vitro reversible inhibition of thrombin catalytic activityMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 7nMAssay Description:Concentration of the compound required to inhibit Human alpha-thrombin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 8.20nMAssay Description:Competitive kinetic for human alpha thrombin inhibition Ki was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 22nMAssay Description:Binding affinity for thrombin was reportedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 260nMAssay Description:Compound was evaluated for inhibition of human alpha-thrombin catalytic activityMore data for this Ligand-Target Pair
TargetSerine protease 1(Bos taurus (bovine))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.00800nMAssay Description:Concentration of the compound required to inhibit Trypsin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.00800nMAssay Description:Concentration of the compound required to inhibit thrombin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.0300nMAssay Description:Concentration of the compound required to inhibit thrombin was determinedMore data for this Ligand-Target Pair
TargetSerine protease 1(Bos taurus (bovine))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.0450nMAssay Description:Concentration of the compound required to inhibit Trypsin was determinedMore data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.230nMAssay Description:Concentration of the compound required to inhibit Plasmin was determinedMore data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Concentration of the compound required to inhibit tissue-type plasminogen activator (t-PA) was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:In vitro inhibitory activity against hydrolysis of thrombin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.60nMAssay Description:Concentration of the compound required to inhibit Coagulation factor X was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:In vitro activity against thrombin was determinedMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:In vitro activity against trypsin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 8.30nMAssay Description:Concentration of the compound required to inhibit Human alpha-thrombin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Concentration of the compound required to inhibit Coagulation factor X was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Concentration of the compound required to inhibit Human alpha-thrombin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Concentration of the compound required to inhibit Human alpha-thrombin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Concentration of the compound required to inhibit Human alpha-thrombin was determinedMore data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Concentration of the compound required to inhibit Plasmin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Inhibitory concentration against thrombin when incubated with the compound for 3 minMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Concentration of the compound required to inhibit Tissue-type plasminogen activator (t-PA) was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rat after 3 min incubation with compoundMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 43nMAssay Description:Concentration of the compound required to inhibit Human alpha-thrombin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 46nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 49nMAssay Description:Inhibitory concentration against thrombin when incubated with the compound for 3 minMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Concentration of the compound required to inhibit Human alpha-thrombin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 72nMAssay Description:Concentration of the compound required to inhibit Human alpha-thrombin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 75nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:In vitro activity against thrombin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:In vitro activity against plasmid was determinedMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:Binding affinity determined by reduction in binding of 125 I-glucagon to the human glucagon receptor expressed on CHO cells in absence of Mg+2More data for this Ligand-Target Pair

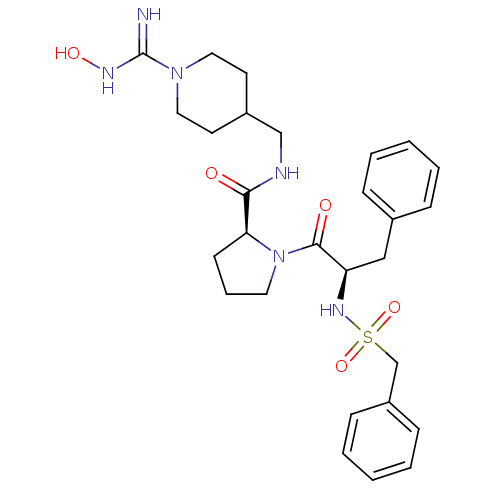

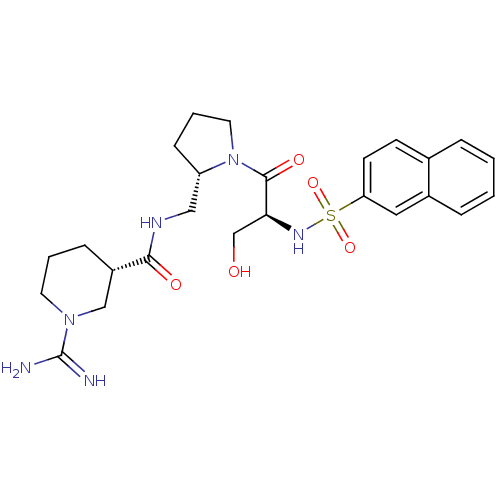
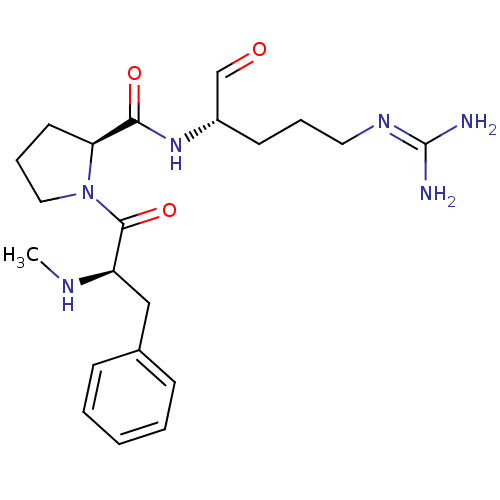
 3D Structure (crystal)
3D Structure (crystal)