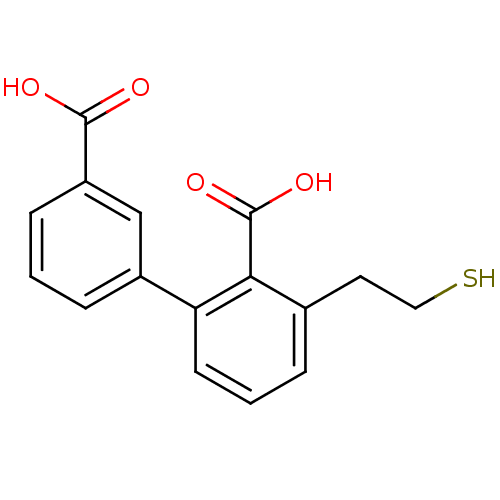
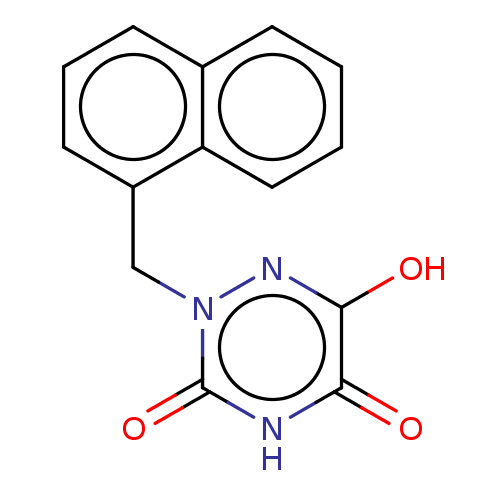
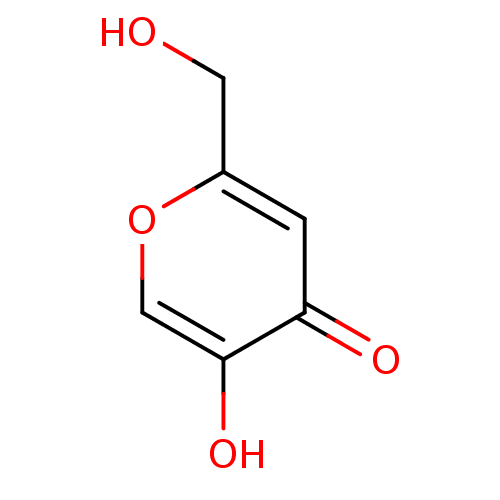
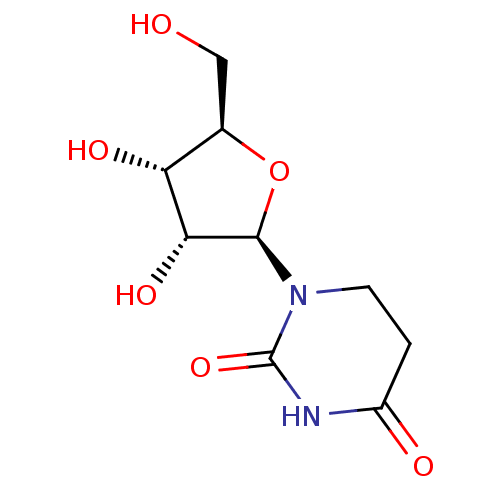
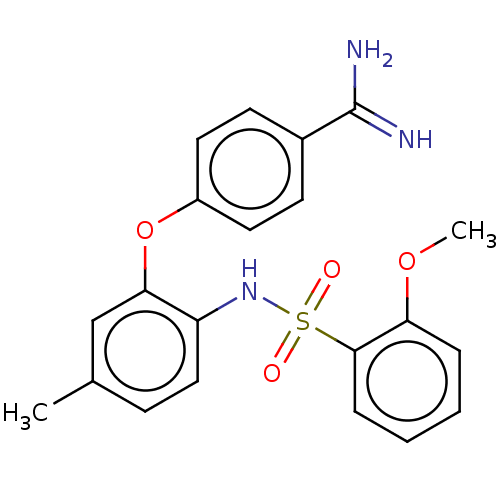
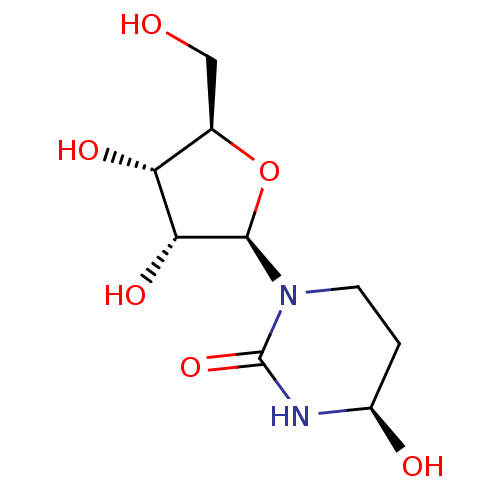
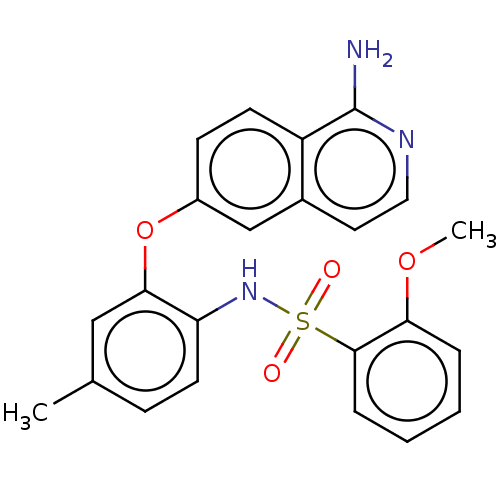
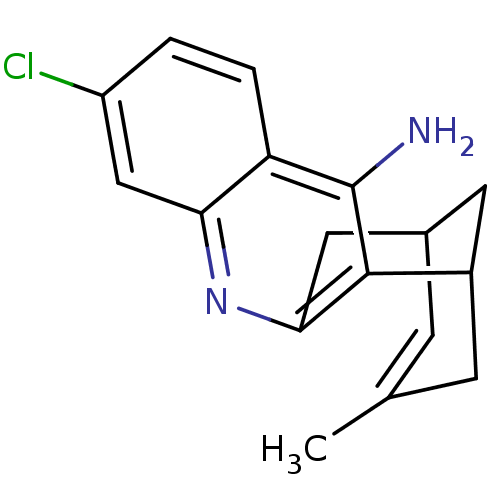
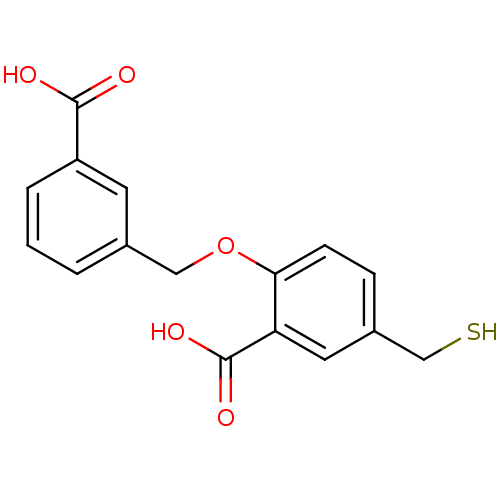
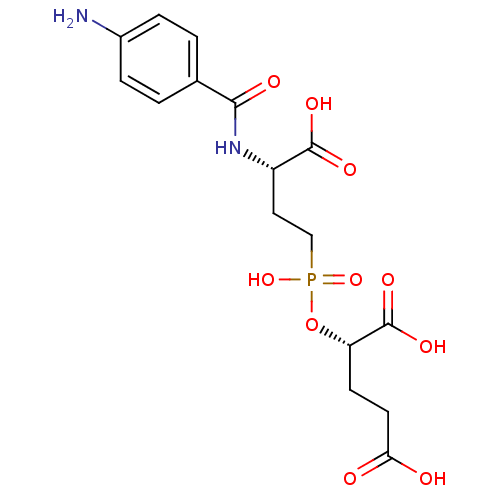
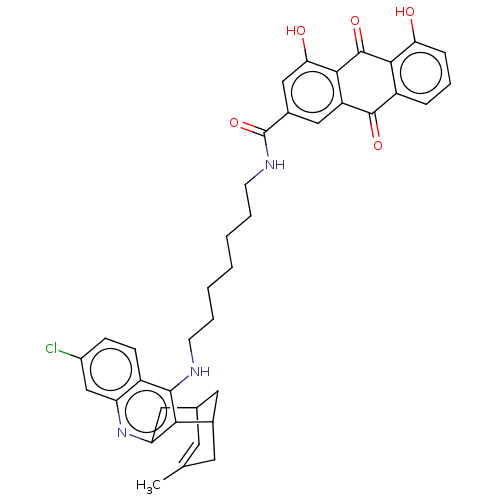
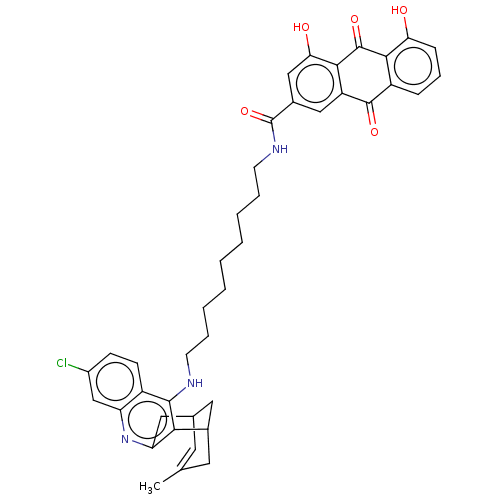
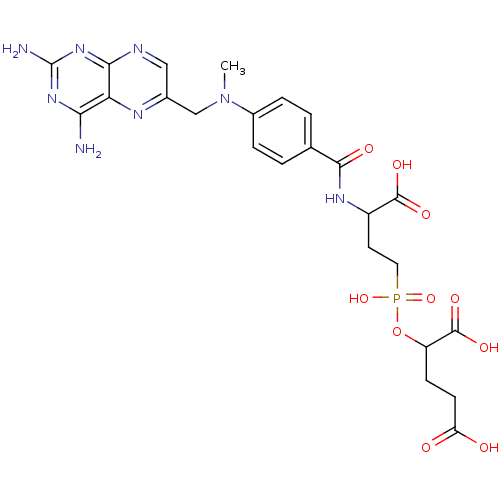
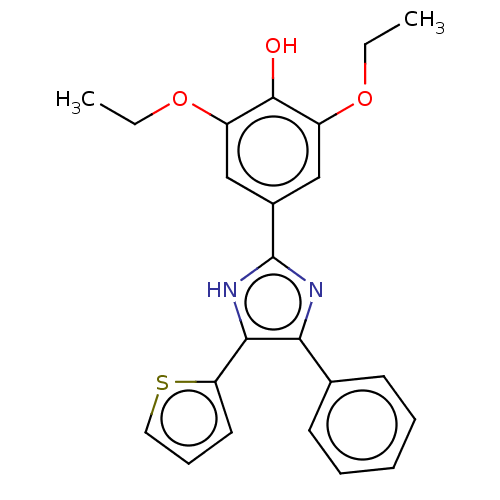
Affinity DataKi: 0.200nMAssay Description:In vitro inhibitory activity against glutamate carboxypeptidase II (GCP II) using N-acetyl-L-aspartyl-[3H]-L-glutamate as a substrateMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Concentration of the compound required for the neuroprotective effect determined by inhibition of GCP IIMore data for this Ligand-Target Pair
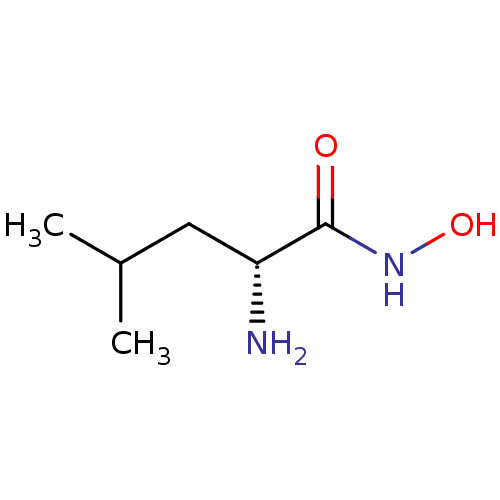
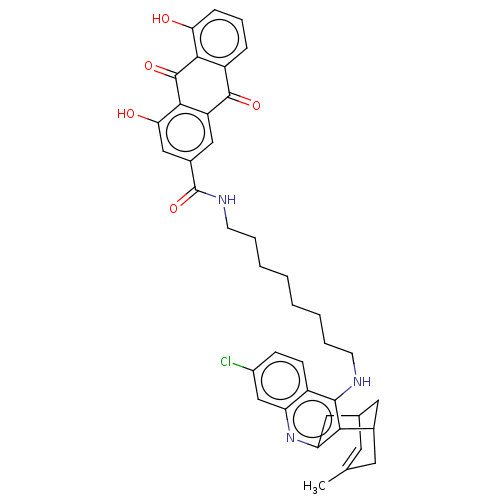
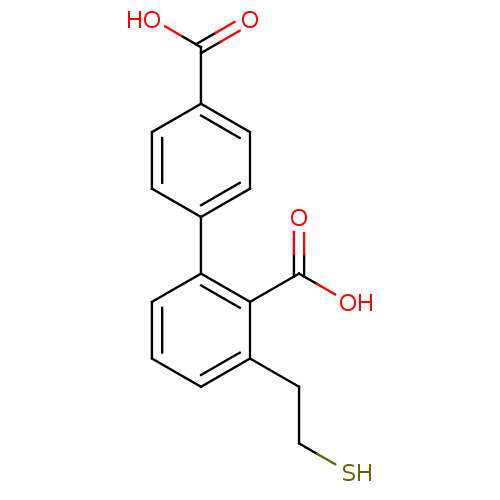
Affinity DataKi: 1nMAssay Description:Inhibition of GCP-2 (unknown origin)More data for this Ligand-Target Pair
TargetBacterial leucyl aminopeptidase(Vibrio proteolyticus)
Guilford Pharmaceuticals
Curated by ChEMBL
Guilford Pharmaceuticals
Curated by ChEMBL
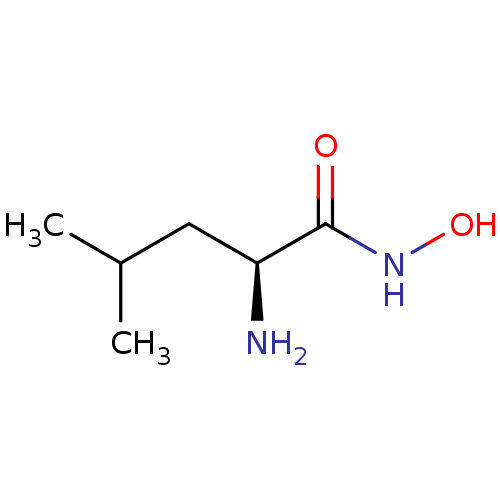
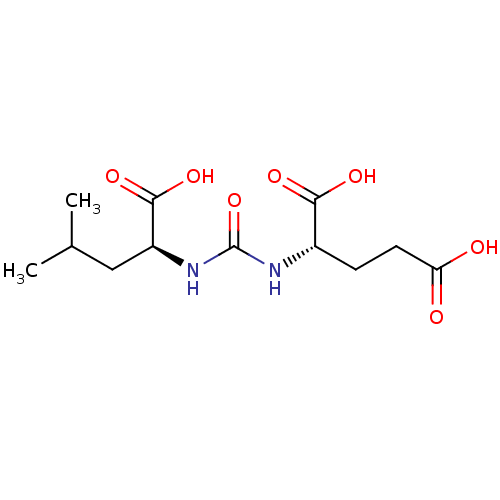
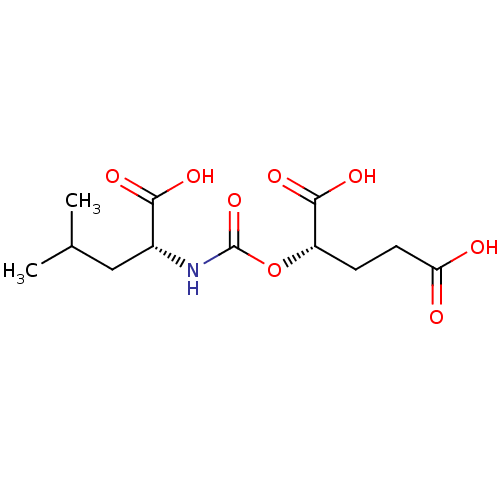
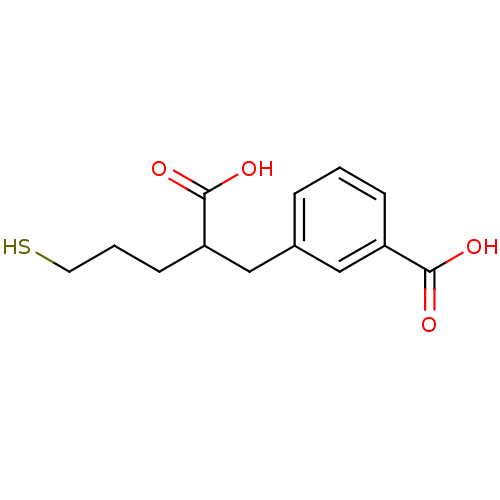
Affinity DataKi: 2nMAssay Description:Inhibition of metalloprotease from family M28, Aeromonas proteolytica aminopeptidaseMore data for this Ligand-Target Pair
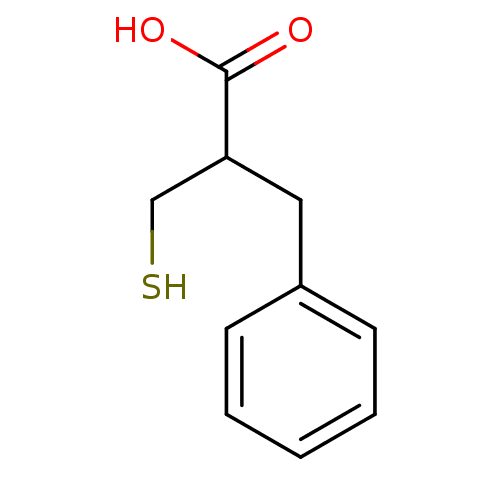
Affinity DataKi: 2.70nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate assessed as dissociation constant for enzyme-inhibitor complex by Li...More data for this Ligand-Target Pair
Affinity DataKi: 3.40nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate assessed as dissociation constant for enzyme-substrate-inhibitor com...More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:In vitro inhibitory activity against glutamate carboxypeptidase II (GCP II) using N-acetyl-L-aspartyl-[3H]-L-glutamate as a substrateMore data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Competitive inhibition of recombinant human DAAO expressed in HEK cells by double reciprocal plot analysis in presence of D-serineMore data for this Ligand-Target Pair
TargetBacterial leucyl aminopeptidase(Vibrio proteolyticus)
Guilford Pharmaceuticals
Curated by ChEMBL
Guilford Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 350nMAssay Description:Inhibition of metalloprotease from family M28, Aeromonas proteolytica aminopeptidaseMore data for this Ligand-Target Pair
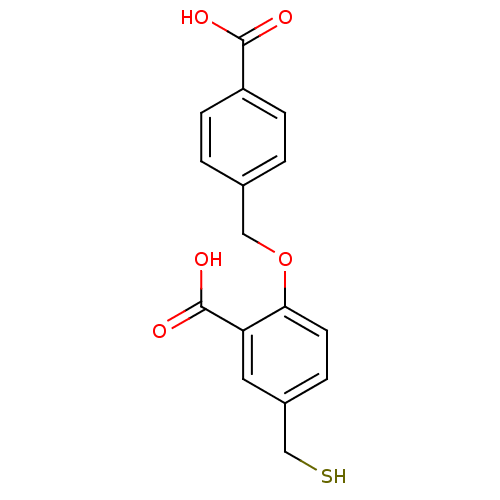
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
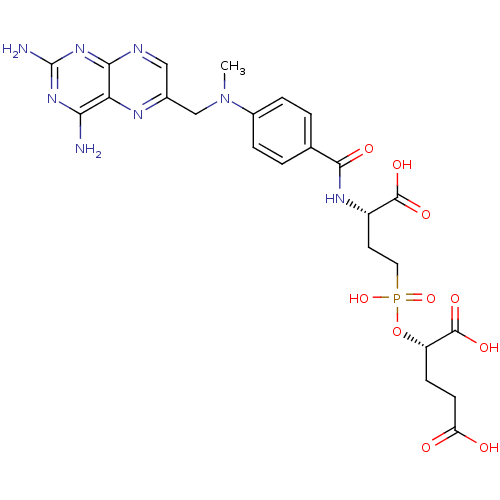
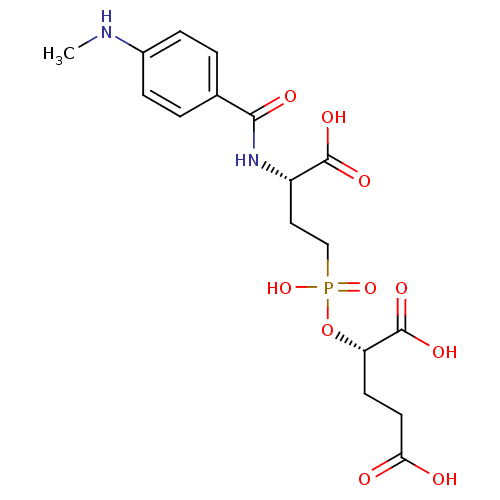
Affinity DataKi: 2.00E+3nMAssay Description:Uncompetitive inhibition of human kidney glutaminase (124 to 669) assessed as reduction of glutamine hydrolysis by double-reciprocal plot analysisMore data for this Ligand-Target Pair
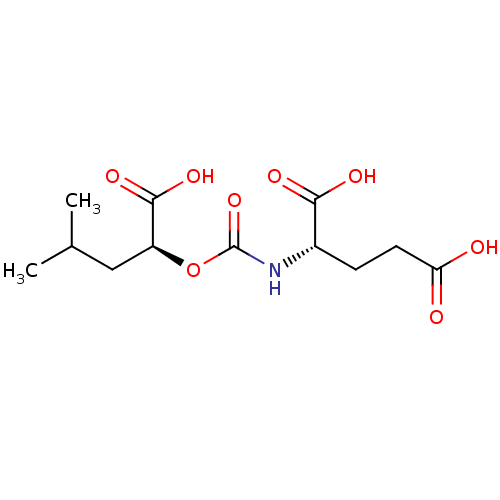
Affinity DataKi: 1.39E+4nMAssay Description:Inhibition of recombinant calmodulin (unknown origin) mediated bovine brain PDE1 activation assessed as effect on inorganic phosphate release using v...More data for this Ligand-Target Pair
Affinity DataKi: 1.93E+4nMAssay Description:Inhibition of recombinant calmodulin (unknown origin) mediated bovine brain PDE1 activation assessed as effect on inorganic phosphate release using v...More data for this Ligand-Target Pair
Affinity DataKi: 2.10E+4nMAssay Description:Competitive inhibition of pig kidney DAAO using D-Alanine as substrate by Michaelis-Menten plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.54E+4nMAssay Description:Inhibition of recombinant calmodulin (unknown origin) mediated bovine brain PDE1 activation assessed as effect on inorganic phosphate release using v...More data for this Ligand-Target Pair
Affinity DataKi: 4.00E+4nMAssay Description:Inhibition of human cytidine deaminase by spectrophotometricallyMore data for this Ligand-Target Pair
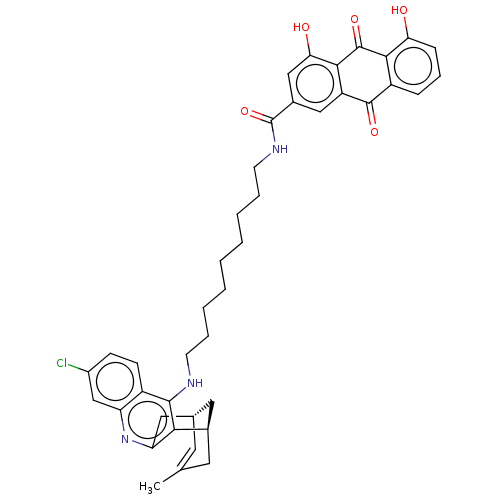
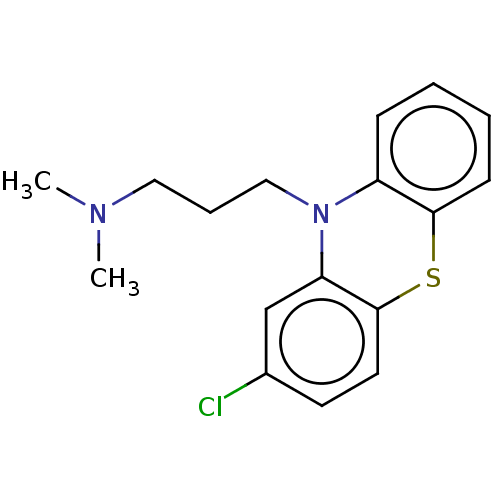
Affinity DataKi: 7.27E+4nMAssay Description:Displacement of [3H]DAMGO from mu opioid receptor in rat brain after 60 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.20E+5nMAssay Description:Displacement of [3H]DADLE from delta opioid receptor in rat brain after 60 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 4.40E+5nMAssay Description:Inhibition of human cytidine deaminase by spectrophotometricallyMore data for this Ligand-Target Pair
Affinity DataKi: 1.43E+6nMAssay Description:Displacement of [3H]U-69,593 from kappa opioid receptor in rat brain after 60 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.47E+6nMAssay Description:Displacement of [3H]DADLE from delta opioid receptor in rat brain after 60 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 3.73E+6nMAssay Description:Displacement of [3H]DAMGO from mu opioid receptor in rat brain after 60 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.21E+7nMAssay Description:Displacement of [3H]U-69,593 from kappa opioid receptor in rat brain after 60 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of Glutamate carboxypeptidase IIMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of N-acetyl-L-aspartyl-[3H]-L-glutamate binding to glutamate carboxypeptidase II (GCP II)More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of GCP-2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei...More data for this Ligand-Target Pair
Affinity DataIC50: 0.650nMAssay Description:Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei...More data for this Ligand-Target Pair
Affinity DataIC50: 0.740nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Compound was evaluated for its ability to inhibit Glutamate carboxypeptidase-II using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Compound was evaluated for its ability to inhibit Glutamate carboxypeptidase-II using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Compound was evaluated for its ability to inhibit Glutamate carboxypeptidase-II using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 4.40nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Compound was evaluated for its ability to inhibit Glutamate carboxypeptidase-II using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.60nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.90nMAssay Description:Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei...More data for this Ligand-Target Pair
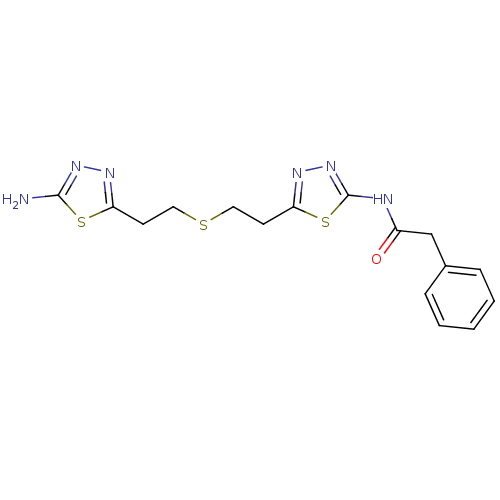
Affinity DataIC50: 10nMAssay Description:Inhibition of human recombinant nSMase expressed in HEK293 cells using sphingomyelin as substrate by alkaline phosphatase, choline oxidase and horser...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's methodMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)