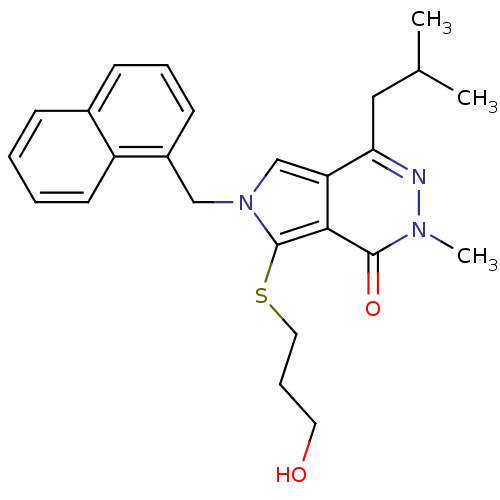
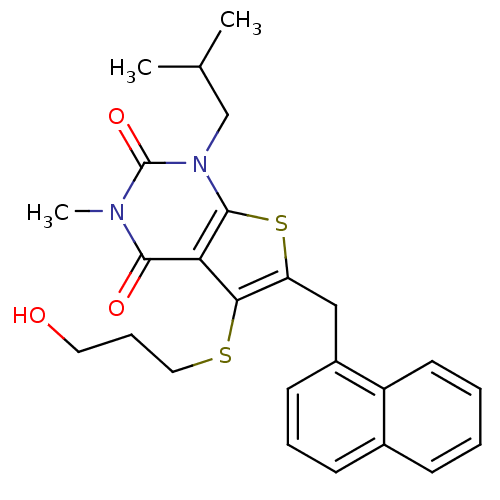
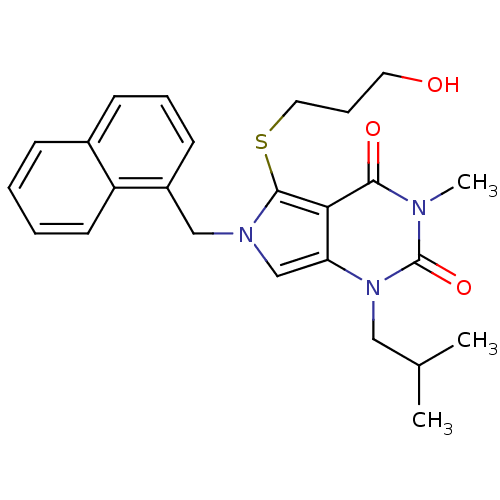
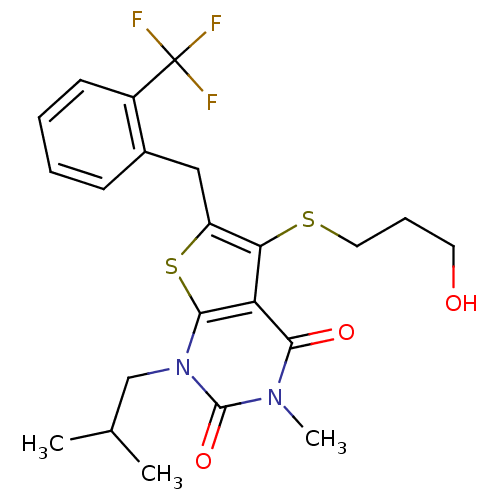
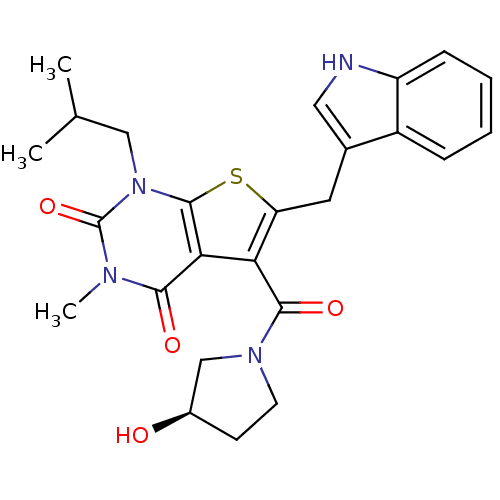
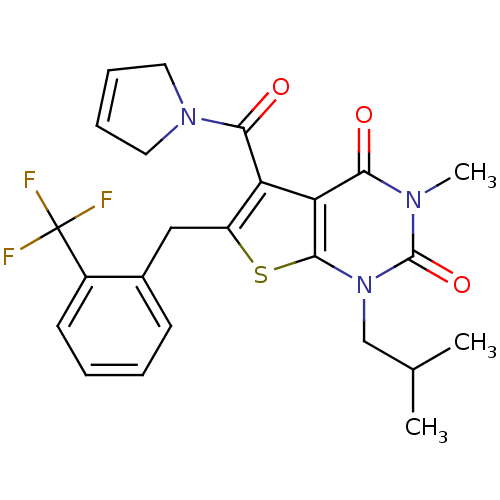
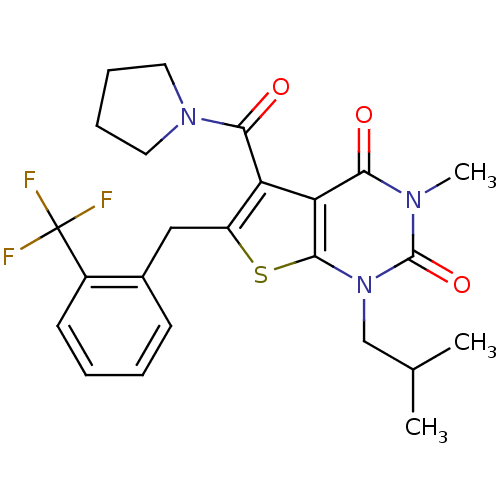
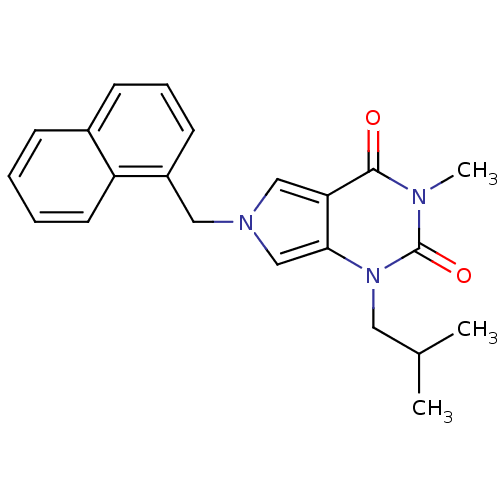
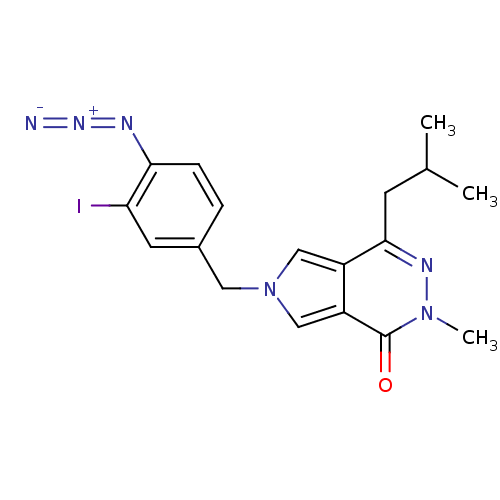
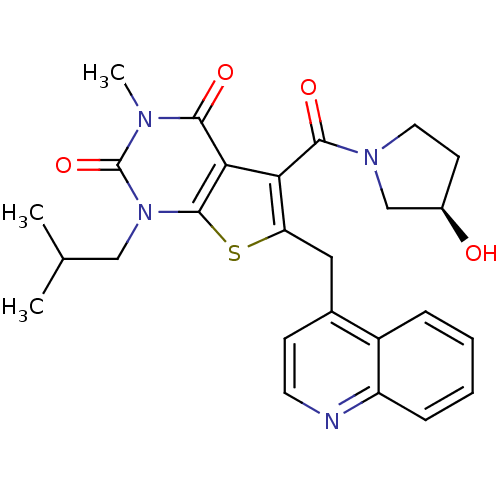
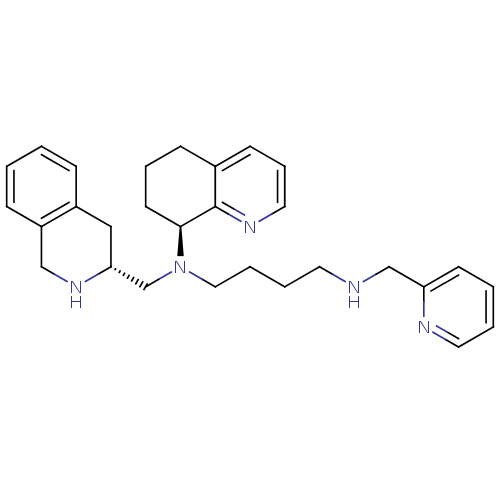
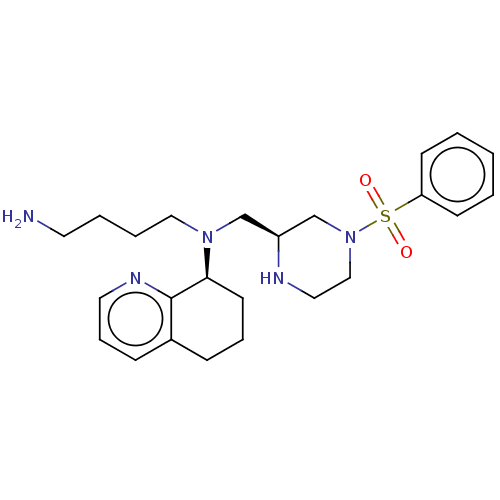
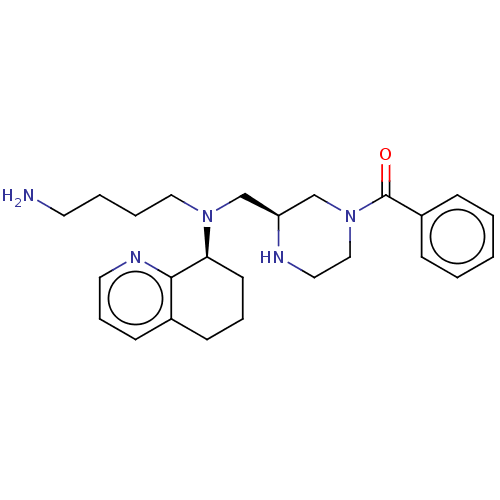
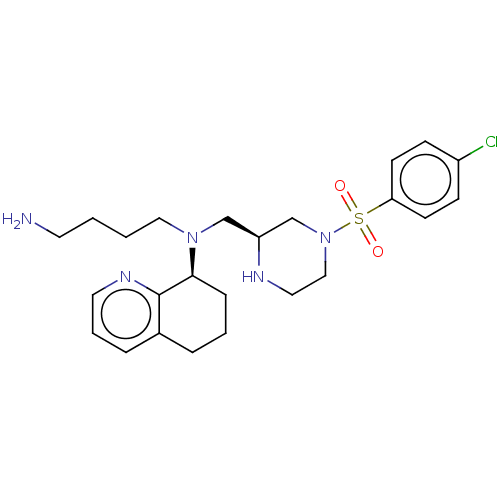
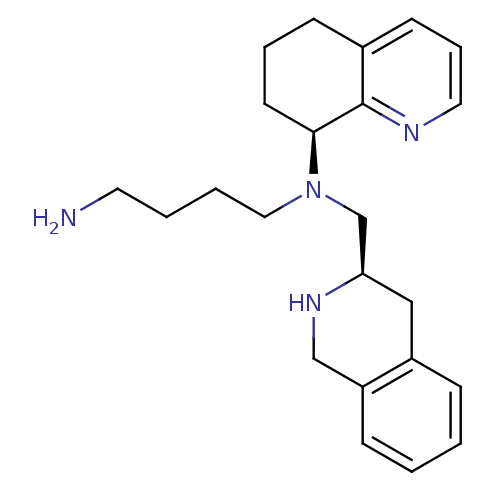
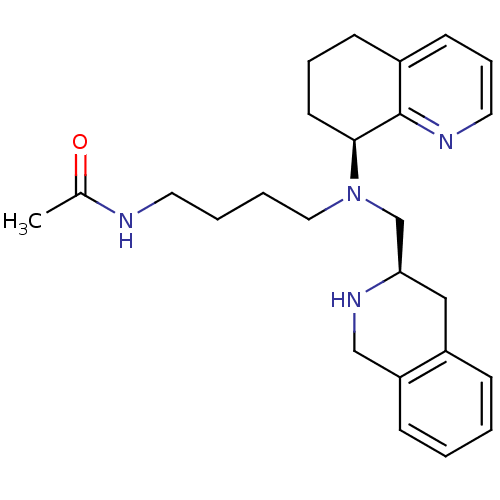
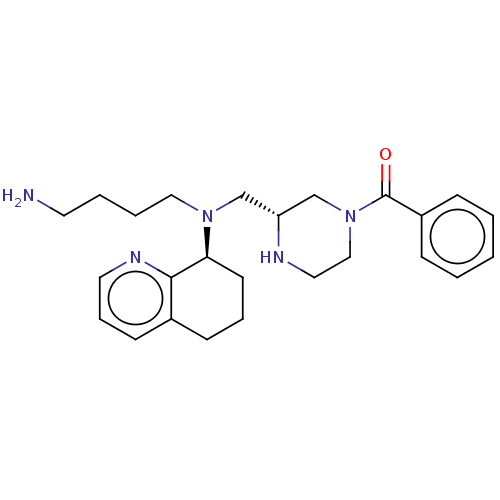
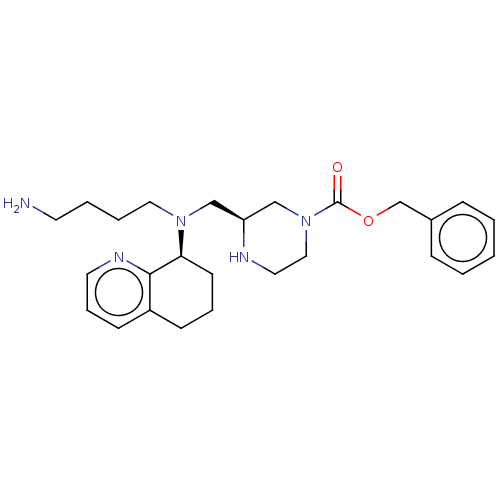
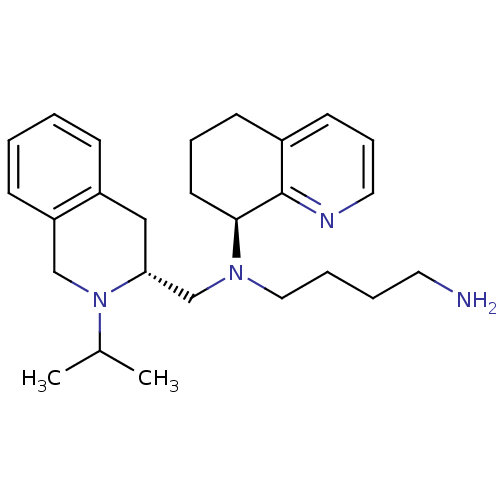
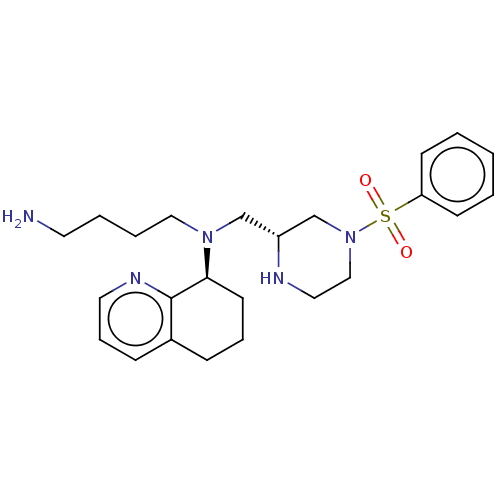
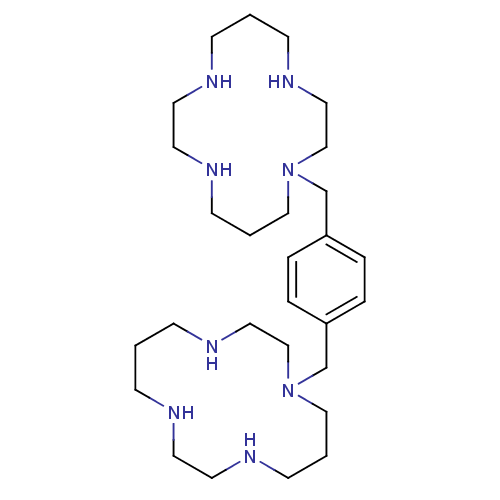
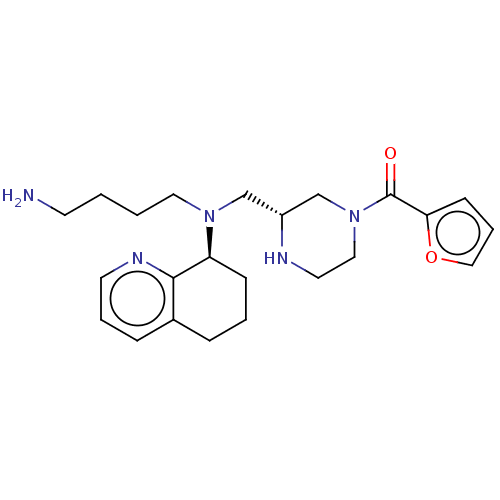
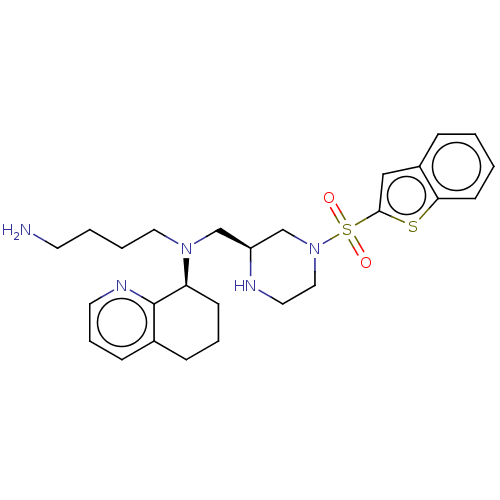
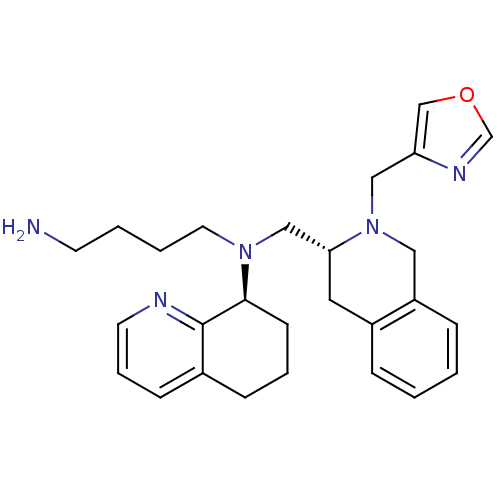
Affinity DataKi: 0.0955nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: 0.275nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: 0.302nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: 0.330nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: 0.331nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: 0.355nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: 0.355nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: 0.708nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: 1.17nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: 3.20nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: 3.39nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: 3.40nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: 5.5nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: 5.60nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: 30.9nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: 31nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: 47nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataKi: <200nMAssay Description:Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Displacement of [125I]-SDF-1 from CXCR4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Displacement of [125I]-SDF-1 from CXCR4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Displacement of [125I]-SDF-1 from CXCR4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Displacement of [125I]-SDF-1 from CXCR4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Antagonist activity at CXCR4 (unknown origin) assessed as inhibition of SDF-1-induced beta-arrestin recruitment incubated for 30 mins prior to SDF-1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Displacement of [125I]-SDF-1 from CXCR4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Antagonist activity at CXCR4 (unknown origin) expressed in CHO-K1 cells assessed as inhibition of SDF-1alpha/forskolin-induced cAMP productionMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ...More data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Displacement of [125I]-SDF-1 from CXCR4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Antagonist activity at CXCR4 in human PBMC assessed as inhibition of HIV-1 3B infectionMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Antagonist activity at CXCR4 in human PBMC assessed as inhibition of HIV-1 3B infectionMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ...More data for this Ligand-Target Pair
Affinity DataIC50: 35nMAssay Description:Antagonist activity at CXCR4 in human PBMC assessed as inhibition of HIV-1 3B infectionMore data for this Ligand-Target Pair
Affinity DataIC50: 35nMAssay Description:Displacement of [125I]-SDF-1 from CXCR4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 39nMAssay Description:Displacement of [125I]-SDF-1 from CXCR4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 39nMAssay Description:Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ...More data for this Ligand-Target Pair
Affinity DataIC50: 56nMAssay Description:Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ...More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ...More data for this Ligand-Target Pair