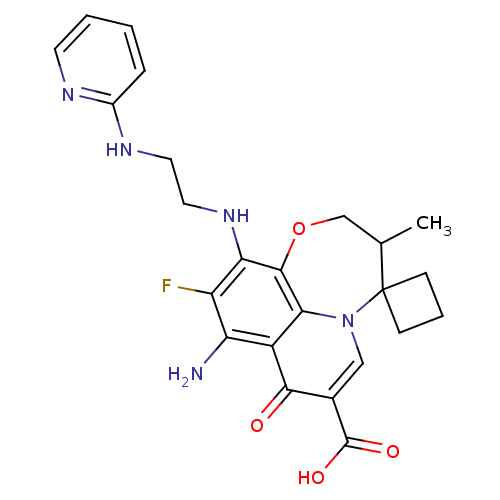
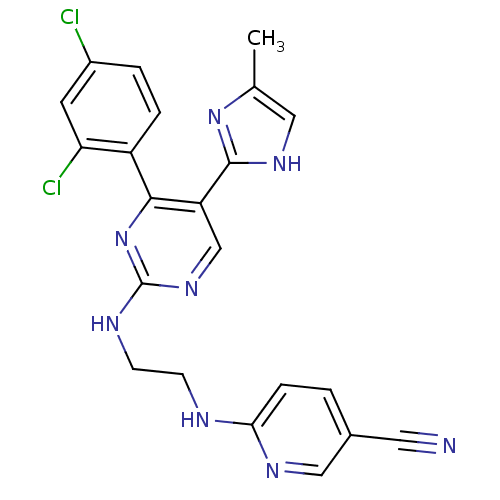
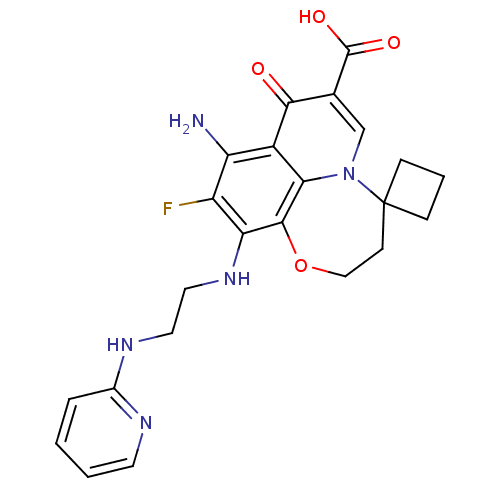
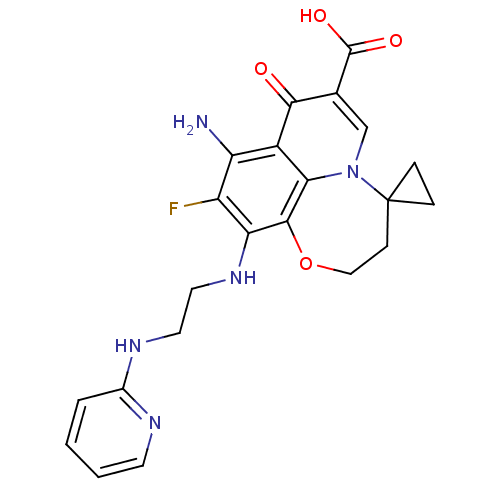
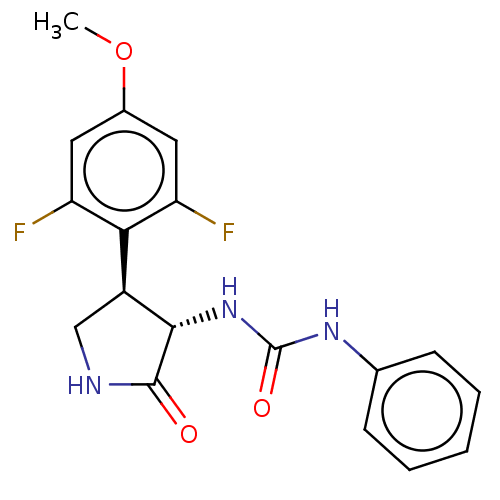
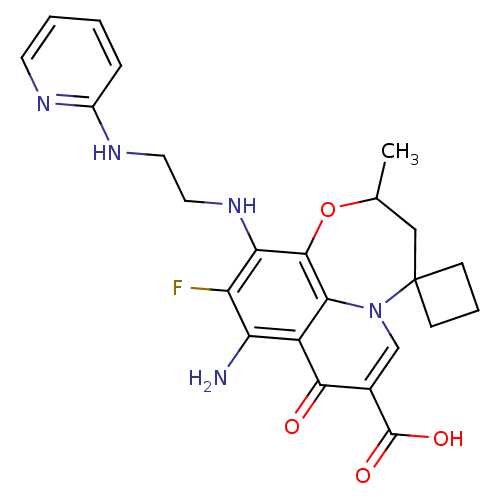
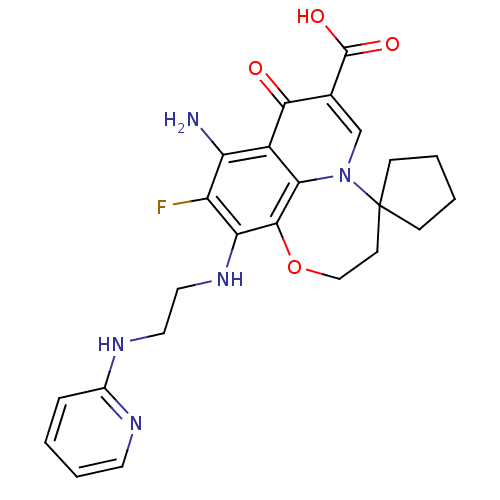
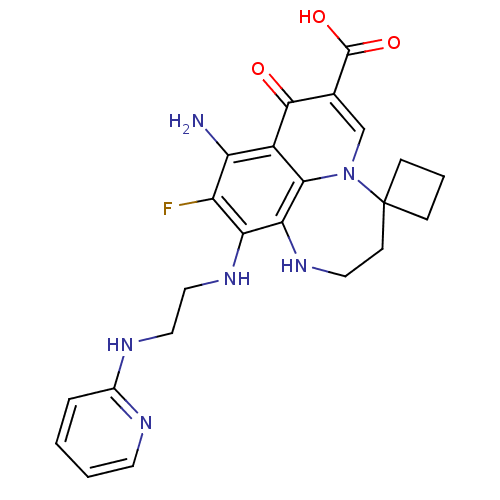
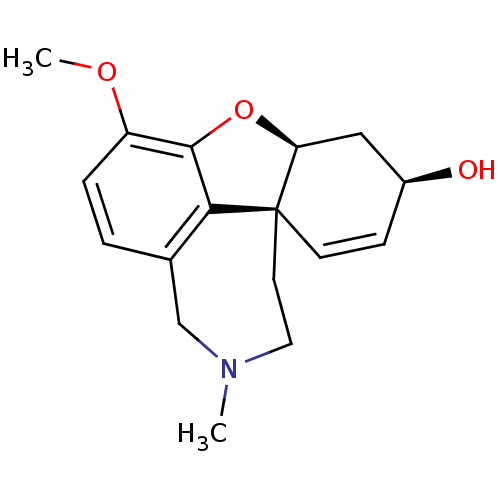
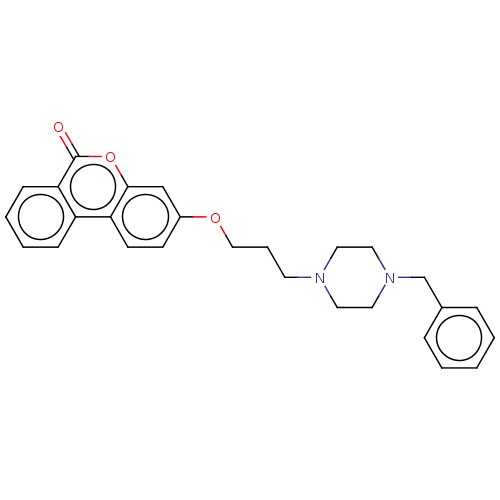
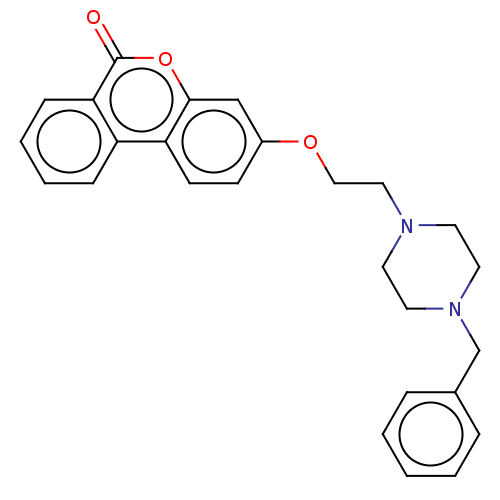
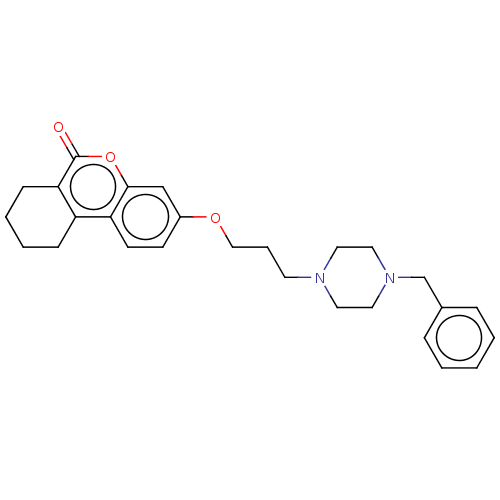
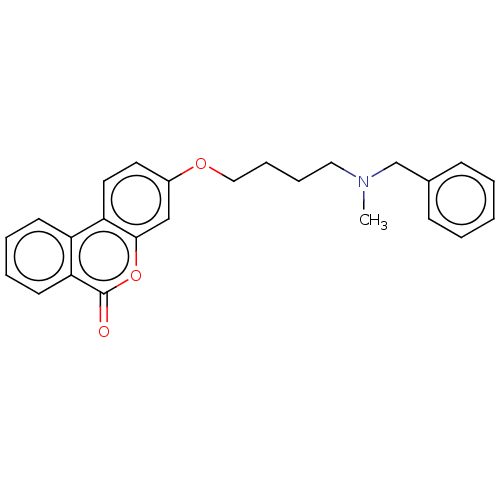
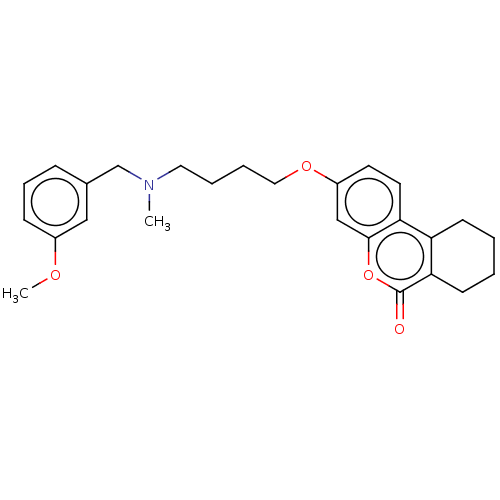
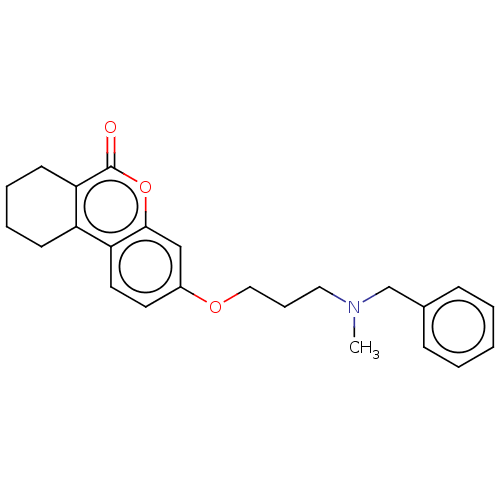
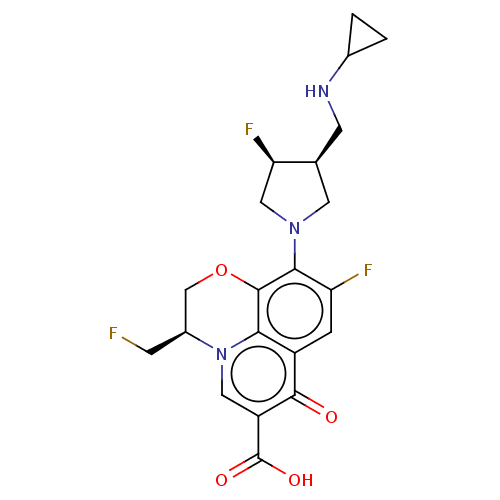
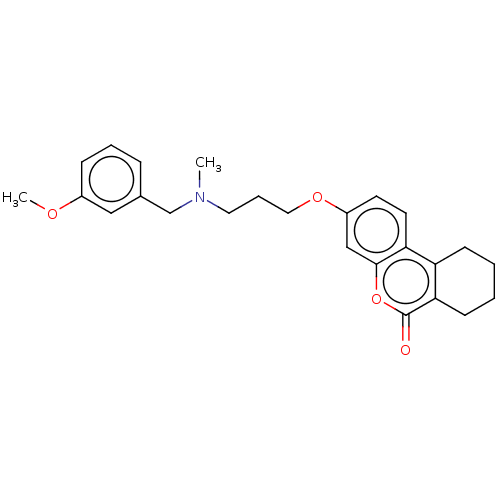
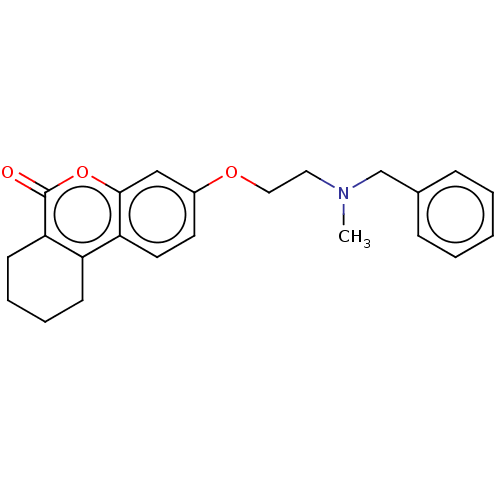
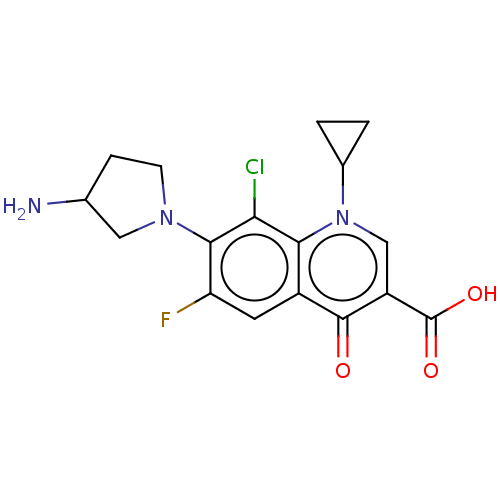
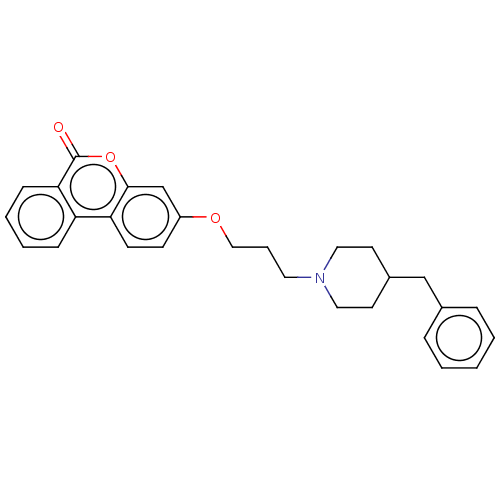
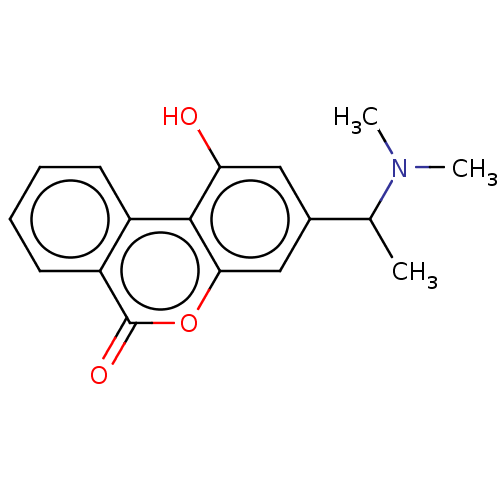
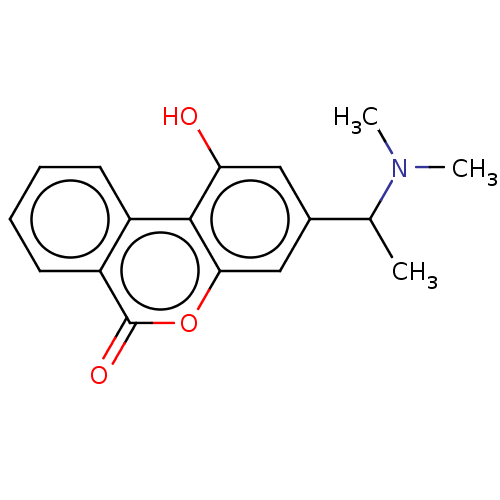
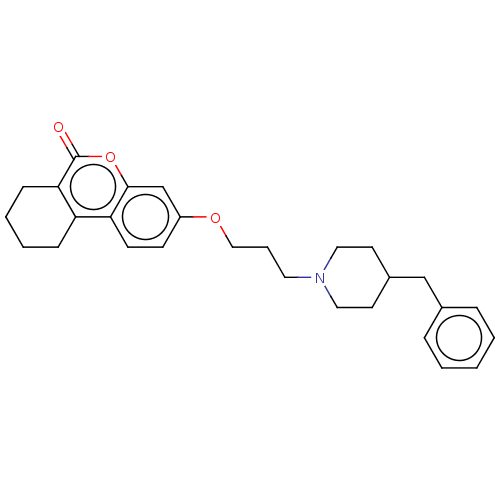
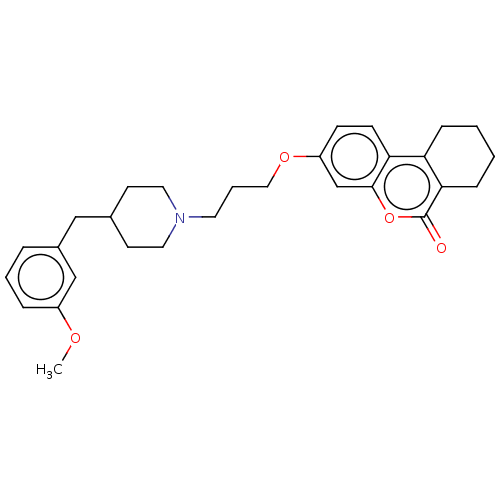
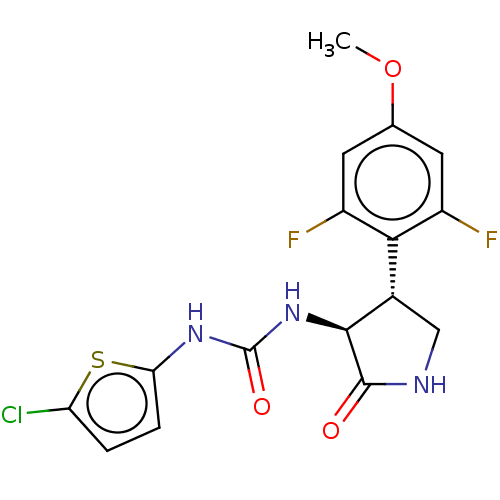
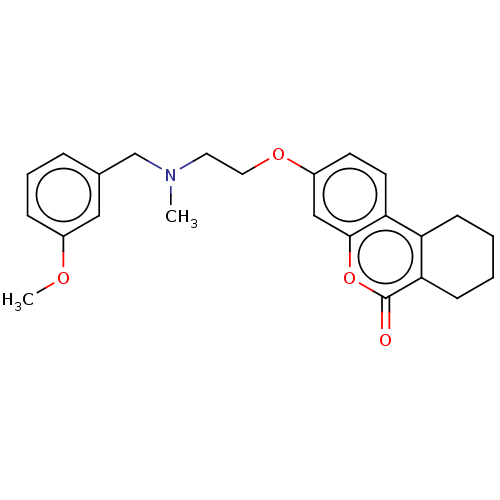
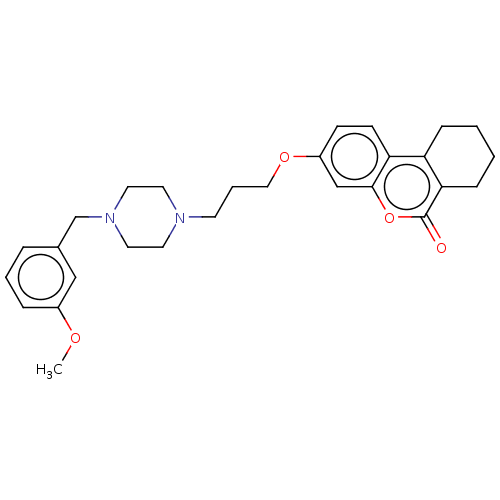
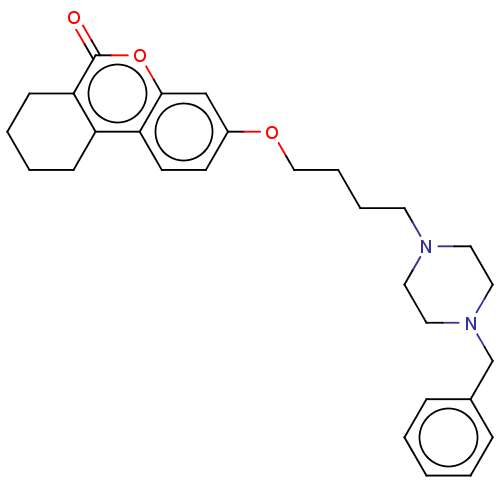
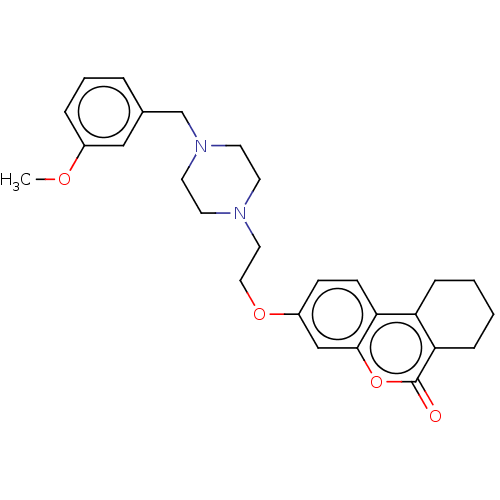
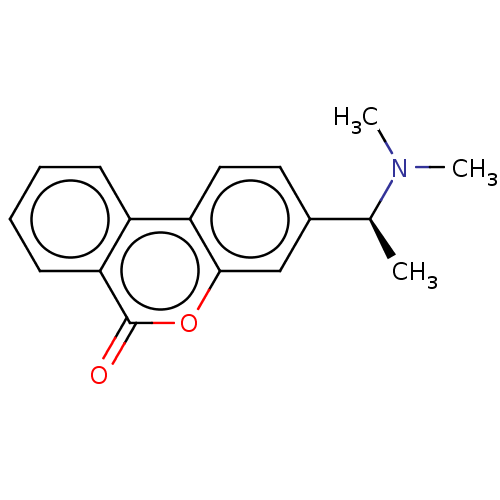
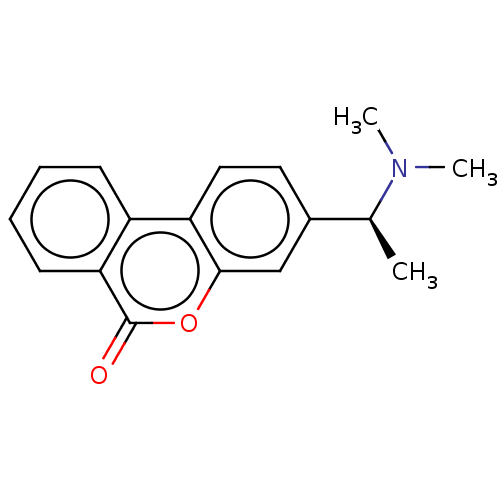
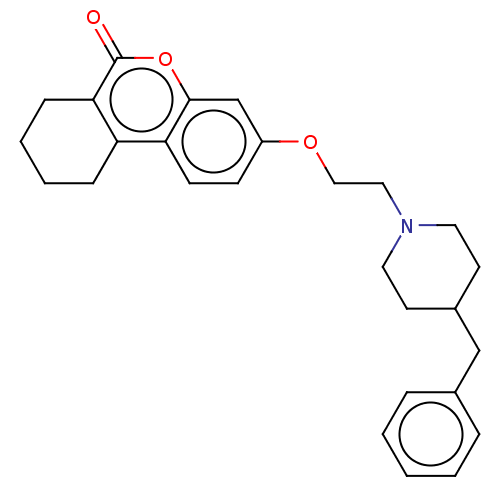
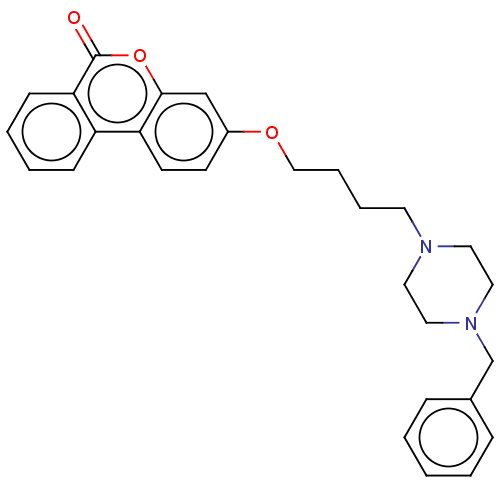
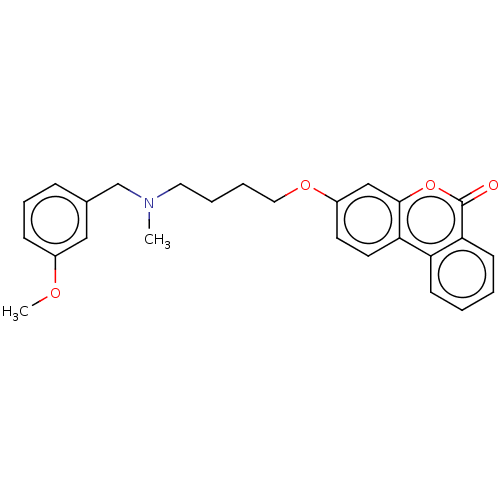
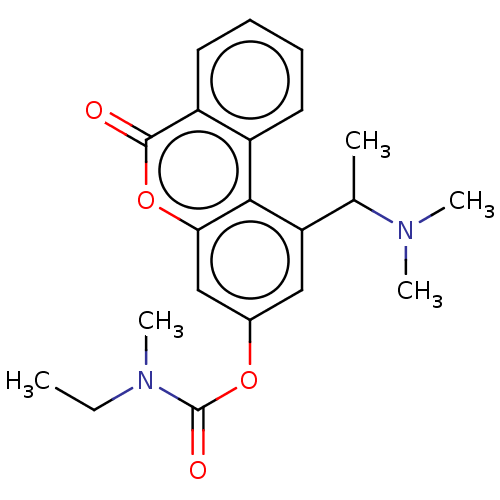
Affinity DataIC50: 8nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human recombinant GSK3-beta after 1 hr by KinaseGlo luciferase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of GSK3-betaMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of human recombinant GSK3-beta after 1 hr by KinaseGlo luciferase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Inhibition of human recombinant GSK3-beta after 1 hr by KinaseGlo luciferase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:Inhibition of human recombinant GSK3-beta after 1 hr by KinaseGlo luciferase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 57nMAssay Description:Agonist activity at FPR2 in human HL-60 cells assessed as reduction in chemoattractant induced chemotaxis by luminescence cell viability assayMore data for this Ligand-Target Pair
Affinity DataIC50: 250nMAssay Description:Inhibition of human recombinant GSK3-beta after 1 hr by KinaseGlo luciferase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 250nMAssay Description:Inhibition of human recombinant GSK3-beta after 1 hr by KinaseGlo luciferase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Inhibition of human recombinant GSK3-beta after 1 hr by KinaseGlo luciferase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 700nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 700nMAssay Description:The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th...More data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.51E+3nMAssay Description:Inhibition of Staphylococcus aureus MS5935 wild type DNA gyrase-mediated supercoiling activityMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th...More data for this Ligand-Target Pair
Affinity DataIC50: 3.10E+3nMAssay Description:Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.28E+3nMAssay Description:Inhibition of Staphylococcus aureus MS5935 wild type DNA gyrase-mediated supercoiling activityMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.20E+3nMAssay Description:Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.20E+3nMAssay Description:The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th...More data for this Ligand-Target Pair
Affinity DataIC50: 4.30E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.90E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.60E+3nMAssay Description:Inhibition of recombinant human CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.20E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.40E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.50E+3nMAssay Description:Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.70E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.10E+3nMAssay Description:The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th...More data for this Ligand-Target Pair
Affinity DataIC50: 7.10E+3nMAssay Description:Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.30E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.90E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.90E+3nMAssay Description:The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th...More data for this Ligand-Target Pair
Affinity DataIC50: 8.10E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.20E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.30E+3nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.50E+3nMAssay Description:Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.50E+3nMAssay Description:The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)