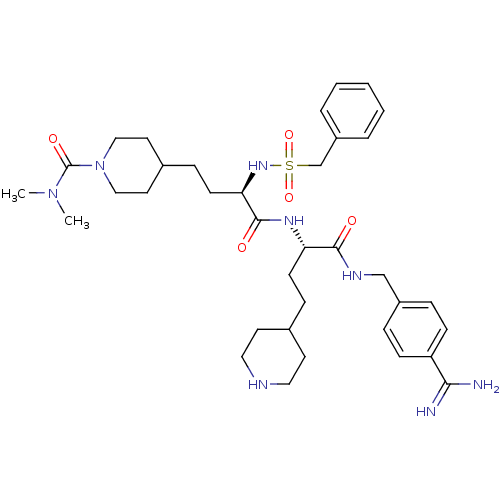
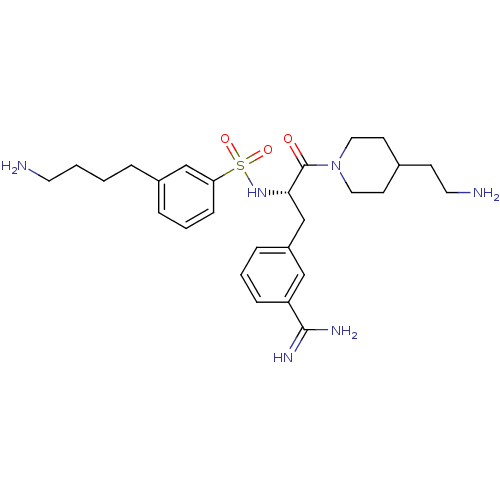
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Curacyte Discovery
Curated by ChEMBL
Curacyte Discovery
Curated by ChEMBL
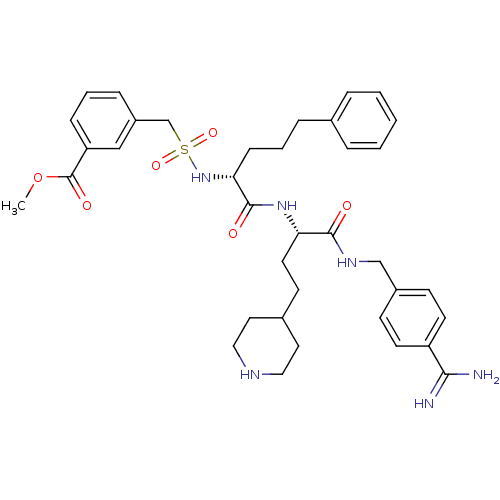
Affinity DataKi: 0.0800nMAssay Description:Inhibition of matriptase (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 0.25nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
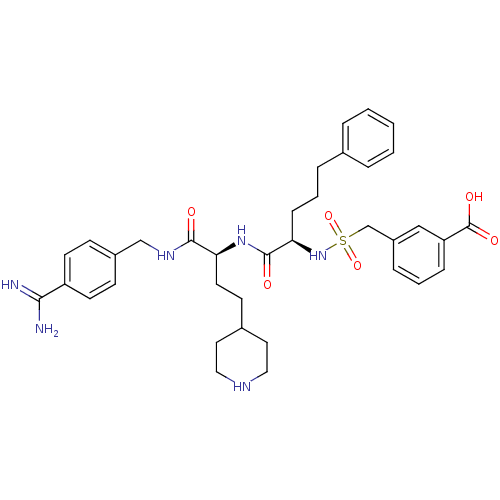
Affinity DataKi: 0.550nMAssay Description:Inhibition of human cathepsin S using Z-Phe-Val-Arg-pNA as substrate after 80 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 0.660nMAssay Description:Inhibition of human cathepsin K using Z-Leu-Arg-AMC as substrate after 80 mins by fluorimetric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
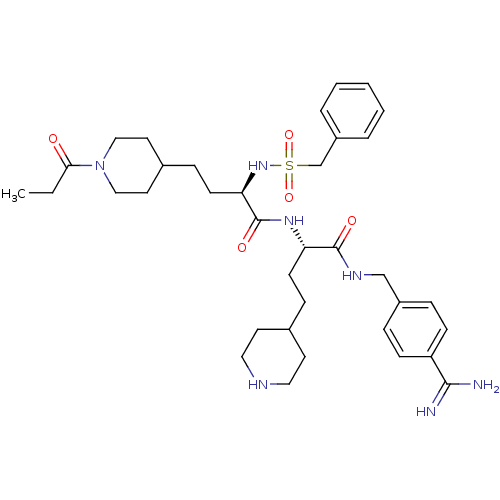
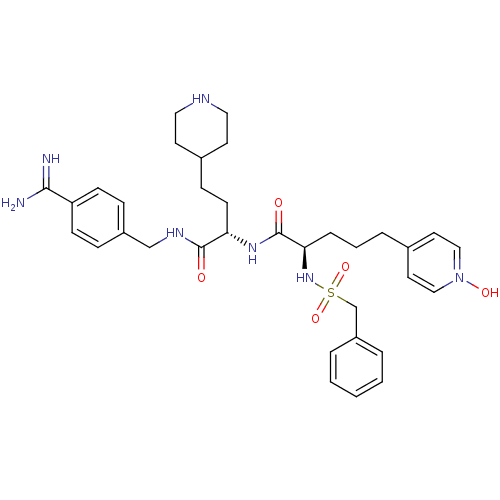
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Curacyte Discovery
Curated by ChEMBL
Curacyte Discovery
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Inhibition of matriptase (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
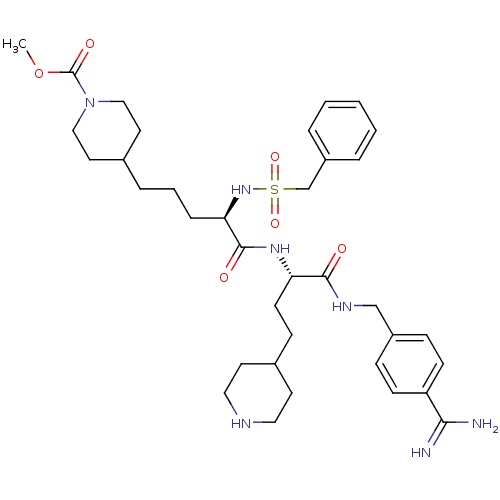
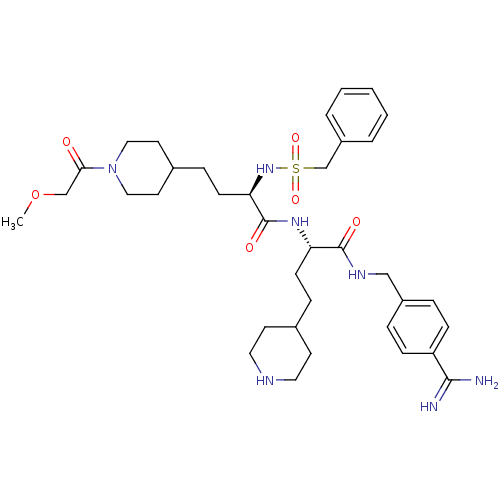
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Curacyte Discovery
Curated by ChEMBL
Curacyte Discovery
Curated by ChEMBL
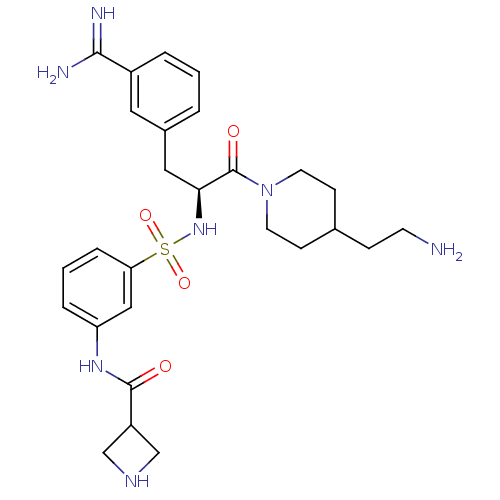
Affinity DataKi: 1.60nMAssay Description:Inhibition of matriptase (unknown origin)More data for this Ligand-Target Pair
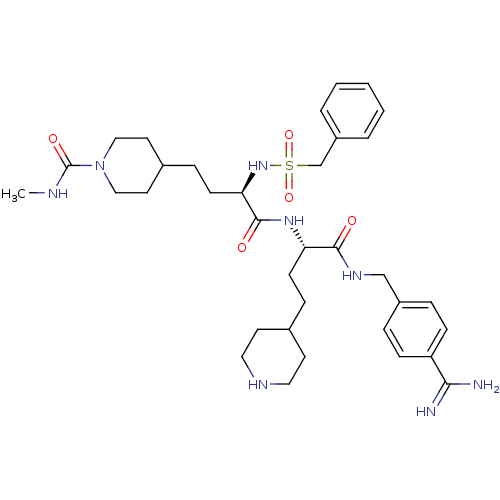
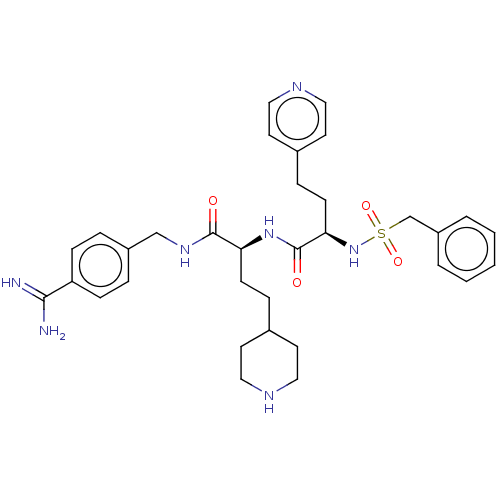
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Curacyte Discovery
Curated by ChEMBL
Curacyte Discovery
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Inhibition of matriptase (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
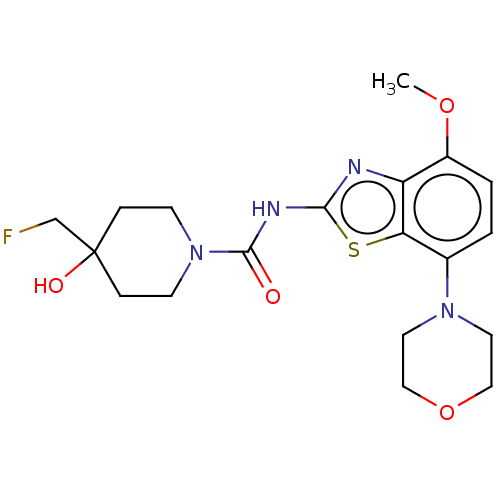
Affinity DataKi: 2.40nMAssay Description:Displacement of [3H]-ZM241385 from recombinant human A2A receptor expressed in CHO-K1 cells incubated for 70 mins by liquid scintillation counting me...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Curacyte Discovery
Curated by ChEMBL
Curacyte Discovery
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Inhibition of matriptase (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.70nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 2.70nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 2.80nMAssay Description:Displacement of [3H]-ZM241385 from recombinant human A2A receptor expressed in CHO-K1 cells incubated for 70 mins by liquid scintillation counting me...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Curacyte Discovery
Curated by ChEMBL
Curacyte Discovery
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Inhibition of matriptase (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 3.60nMAssay Description:Displacement of [3H]-ZM241385 from recombinant human A2A receptor expressed in CHO-K1 cells incubated for 70 mins by liquid scintillation counting me...More data for this Ligand-Target Pair
Affinity DataKi: 3.60nMAssay Description:Displacement of [3H]-ZM241385 from recombinant human A2A receptor expressed in CHO-K1 cells incubated for 70 mins by liquid scintillation counting me...More data for this Ligand-Target Pair
Affinity DataKi: 3.80nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Curacyte Discovery
Curated by ChEMBL
Curacyte Discovery
Curated by ChEMBL
Affinity DataKi: 3.80nMAssay Description:Inhibition of matriptase (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 3.80nMAssay Description:Displacement of [3H]-ZM241385 from recombinant human A2A receptor expressed in CHO-K1 cells incubated for 70 mins by liquid scintillation counting me...More data for this Ligand-Target Pair
Affinity DataKi: 3.90nMAssay Description:Displacement of [3H]-ZM241385 from recombinant human A2A receptor expressed in CHO-K1 cells incubated for 70 mins by liquid scintillation counting me...More data for this Ligand-Target Pair

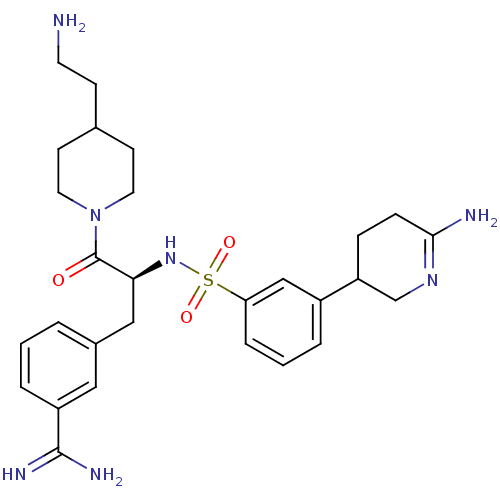
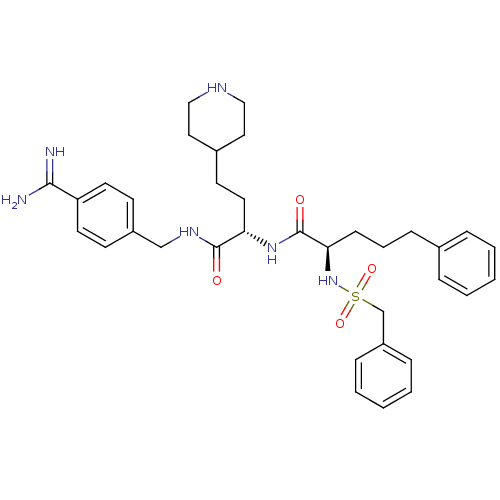
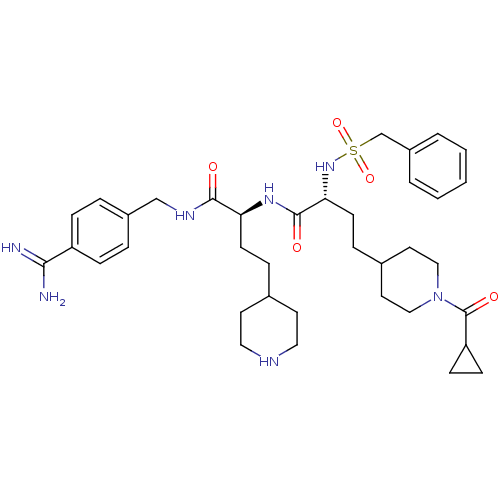
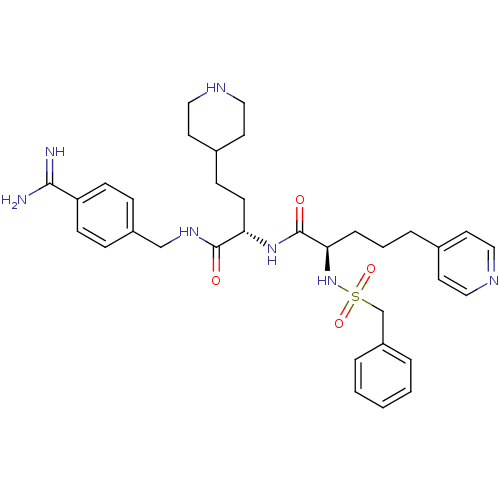
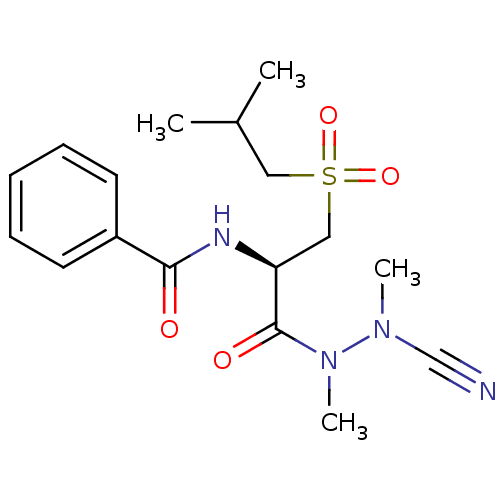
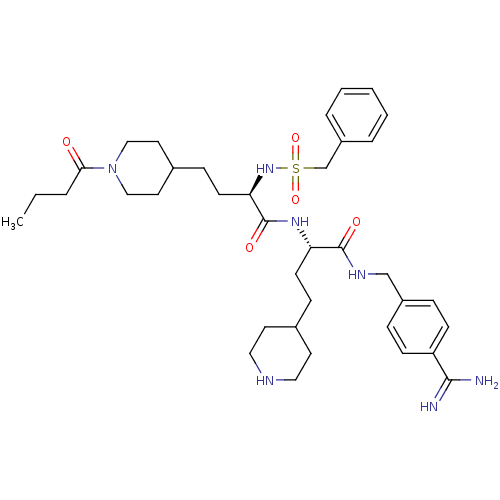
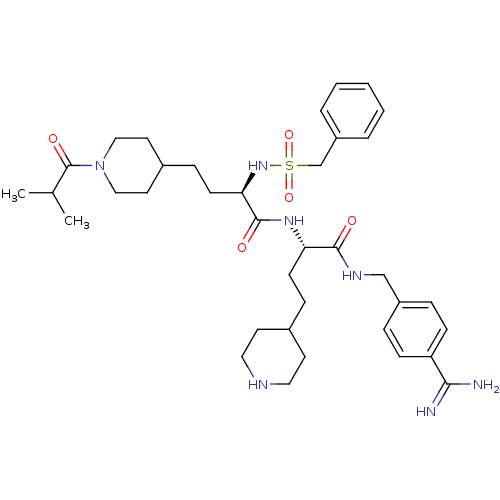
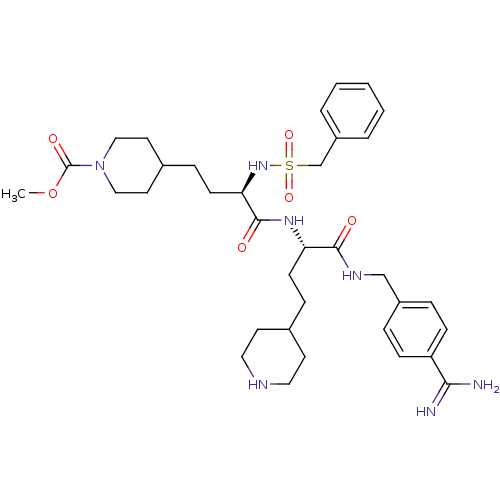
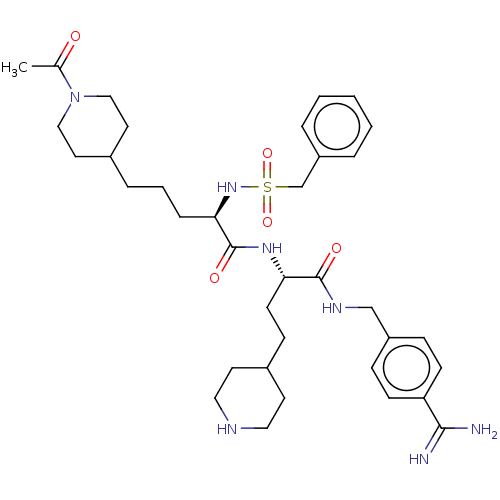
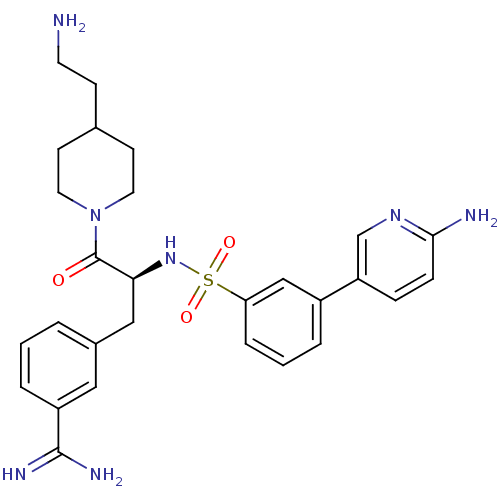
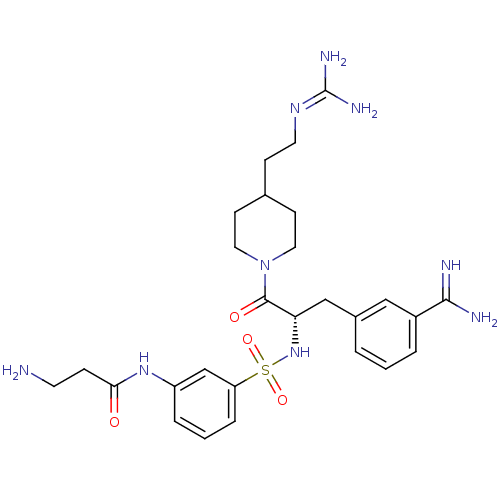
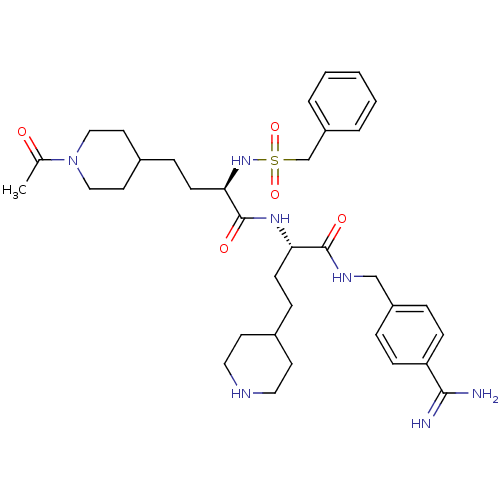
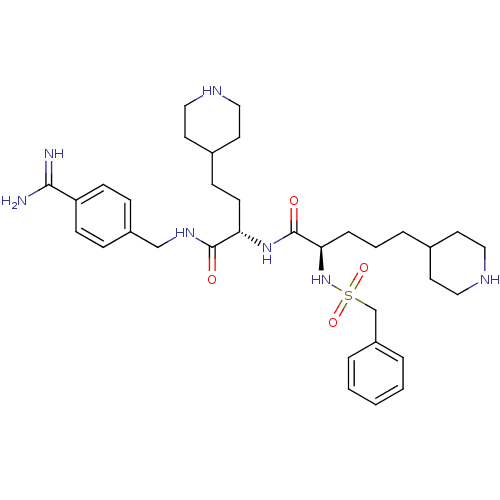
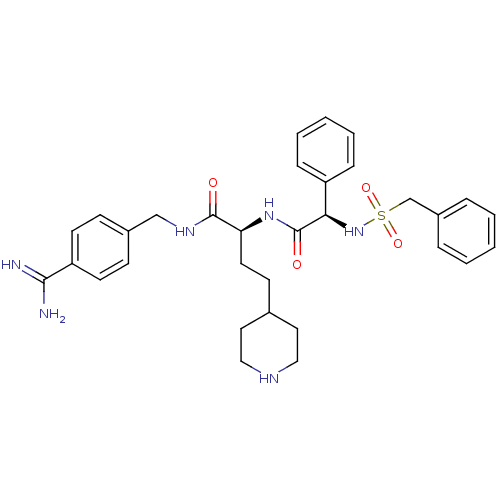
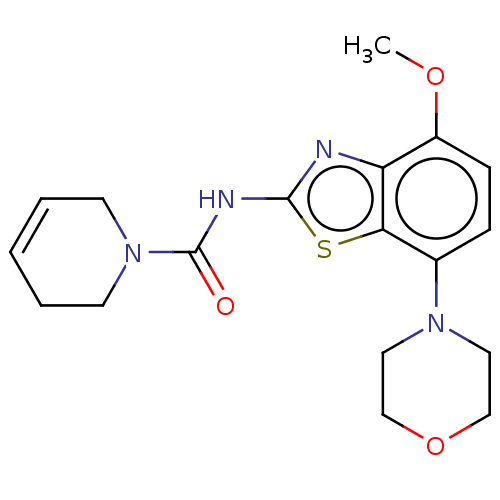
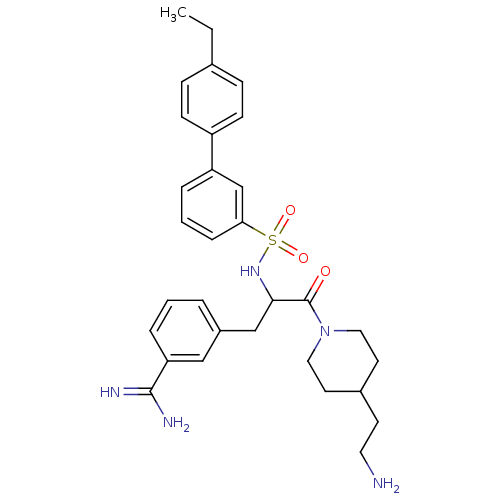

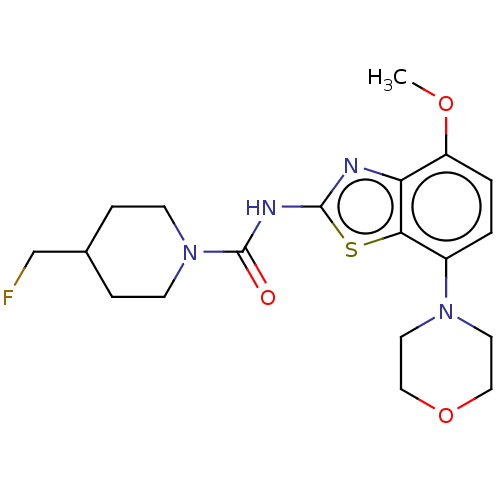
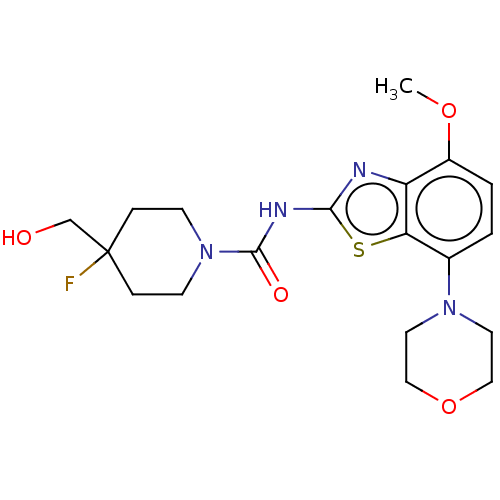
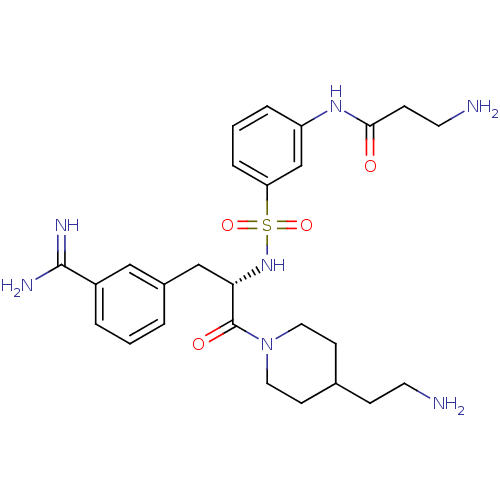
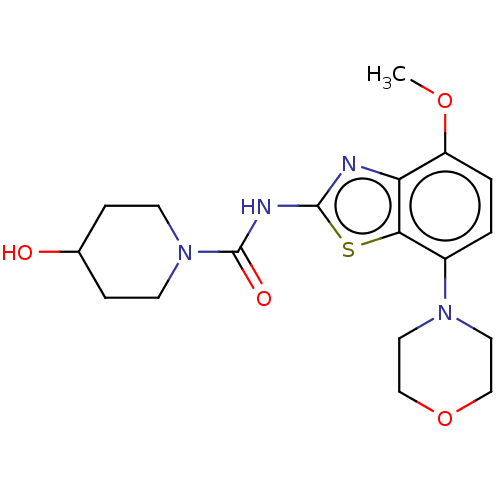
 3D Structure (crystal)
3D Structure (crystal)