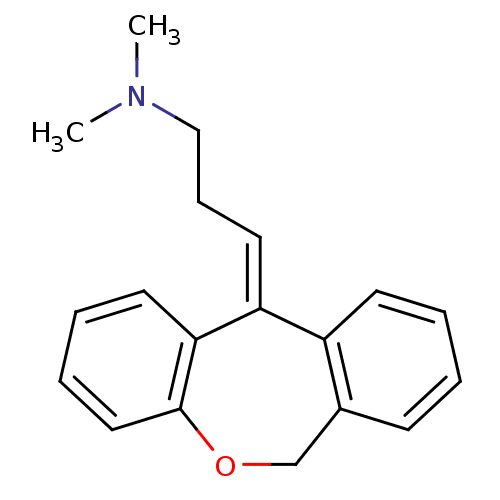
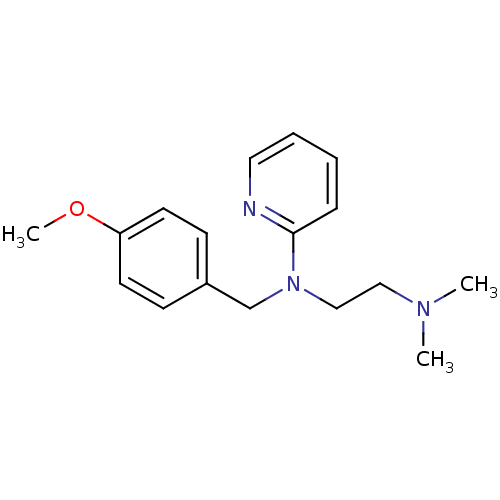
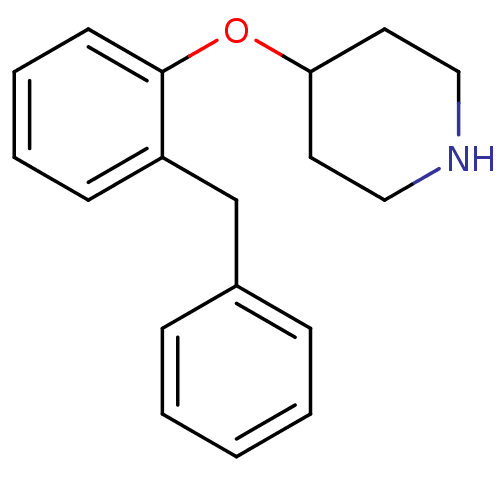
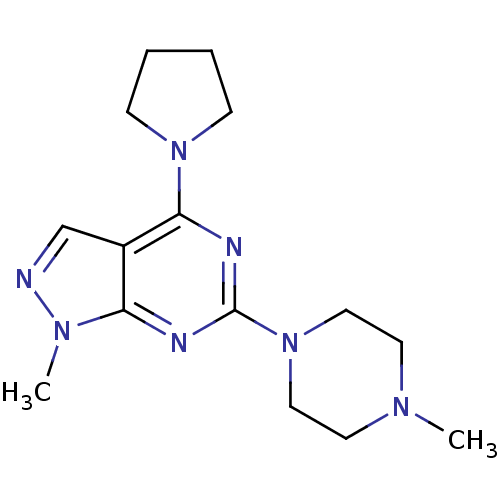
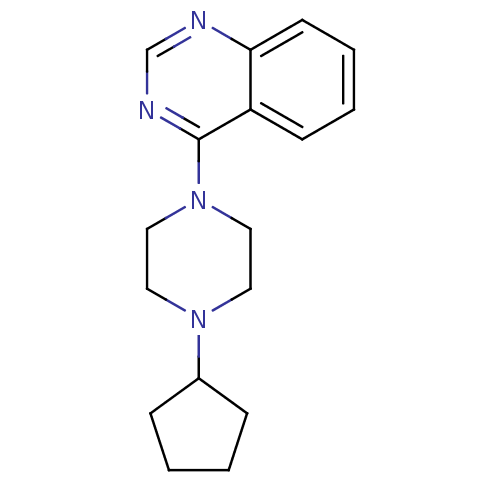
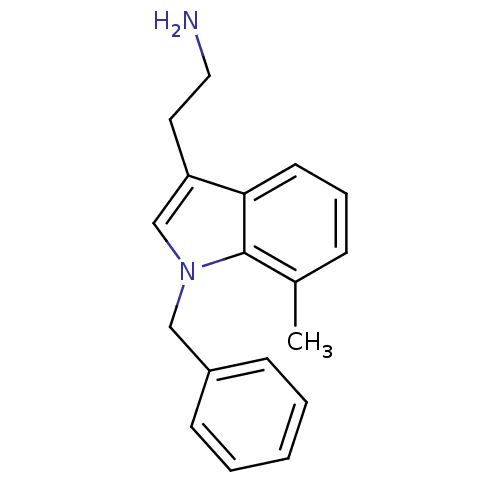
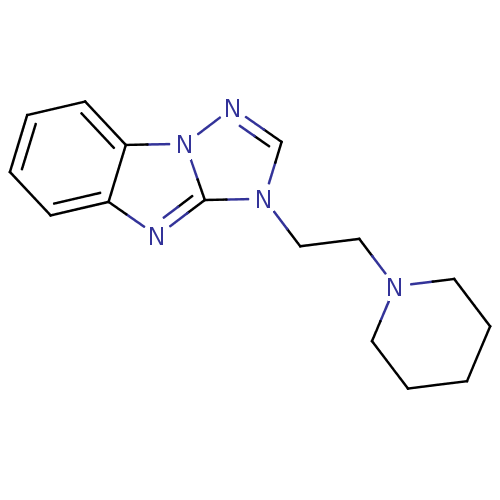
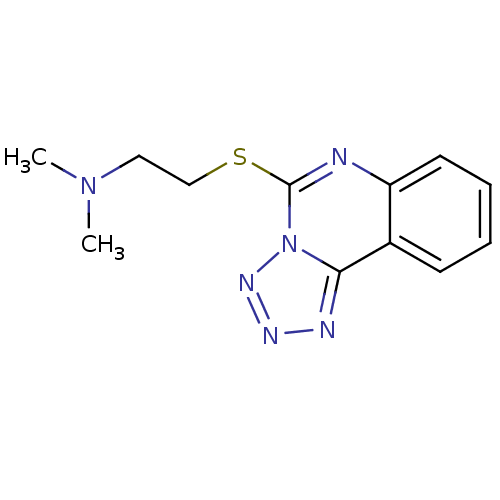
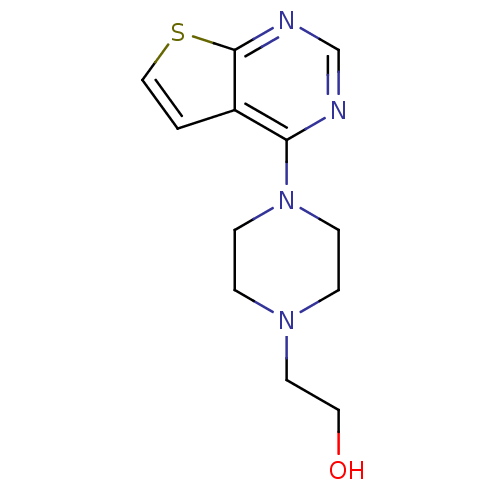
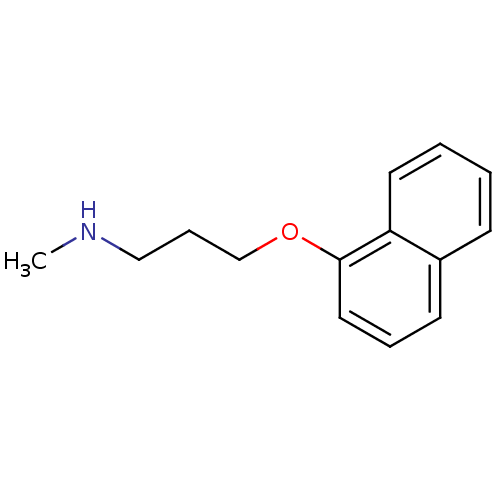
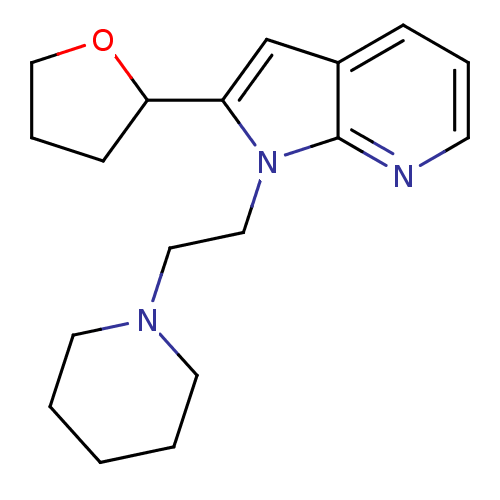
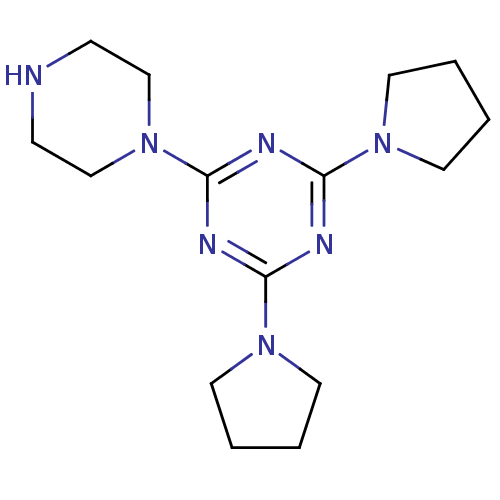
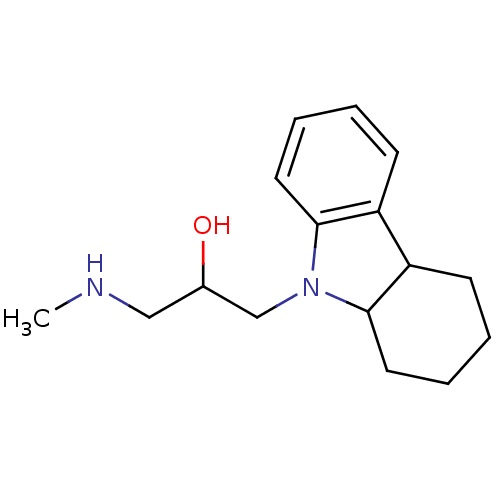
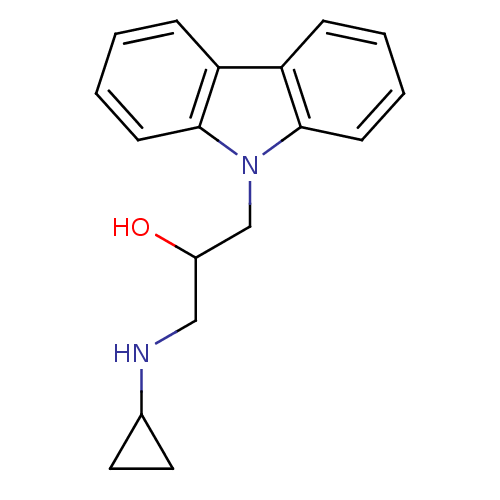
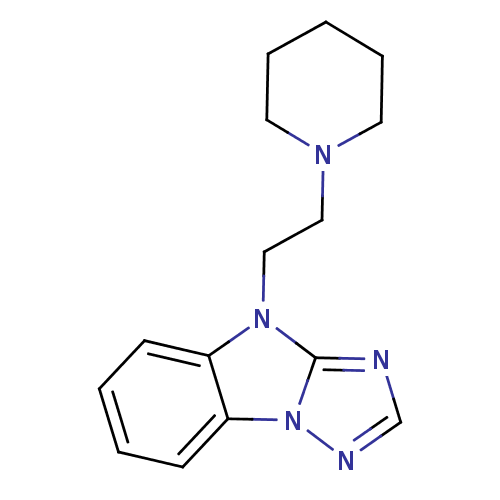
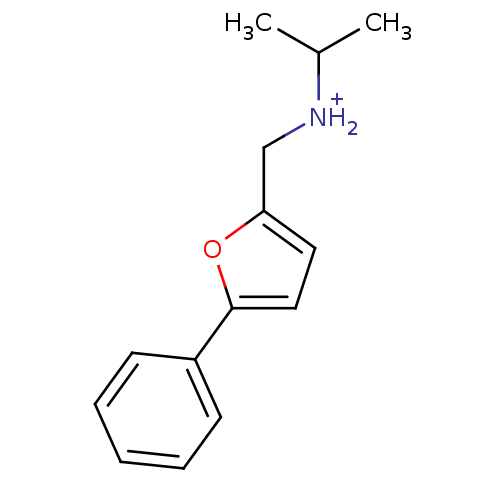
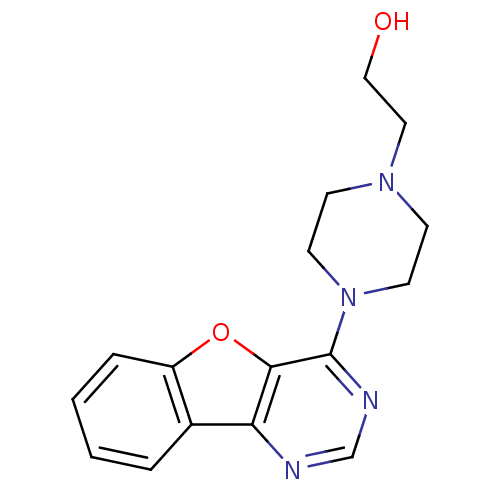
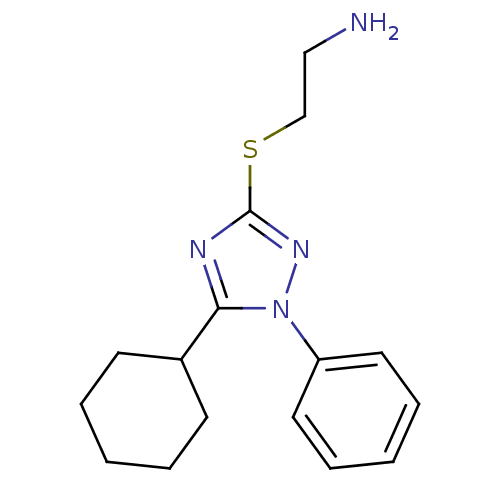
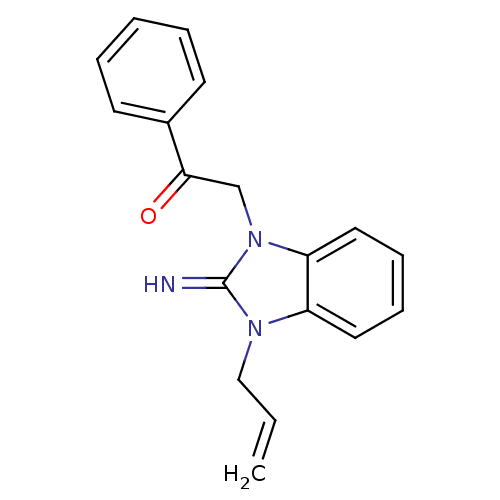
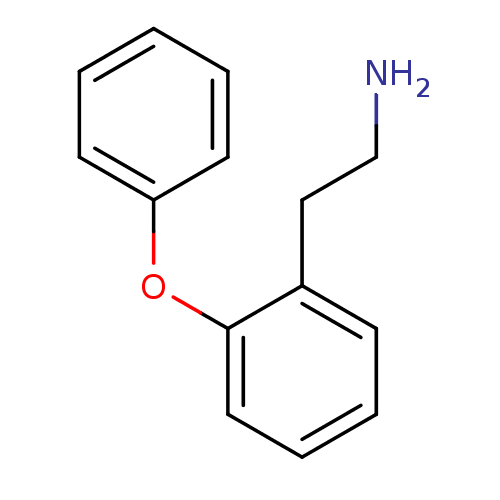
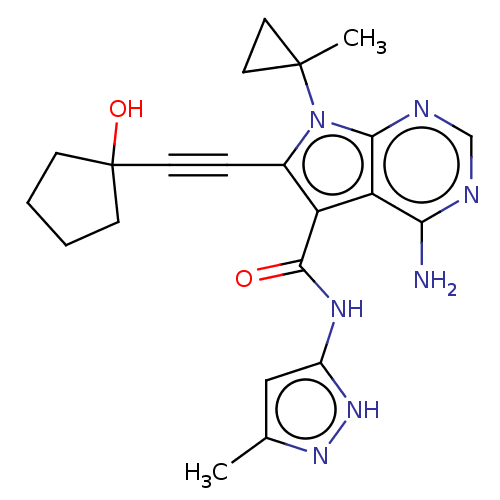
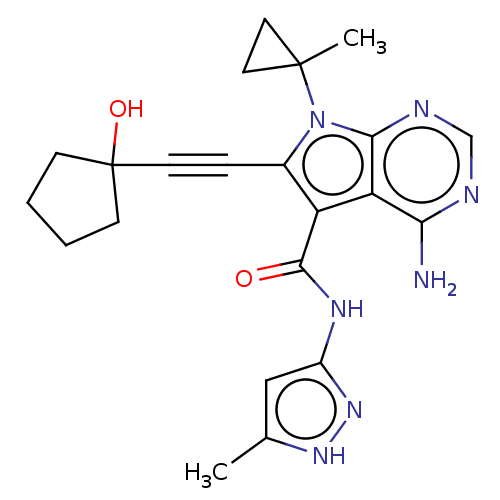
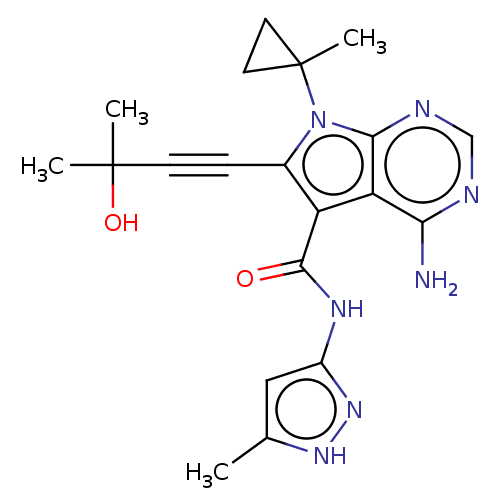
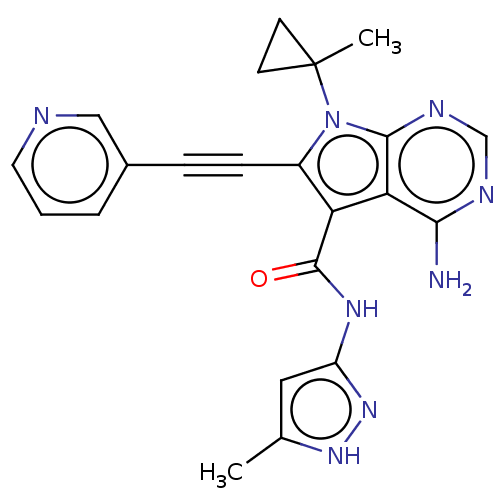
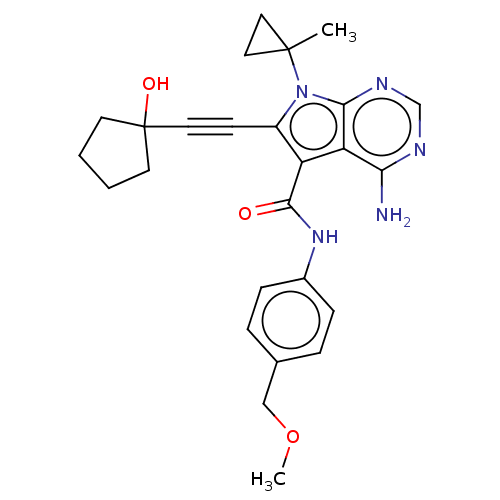
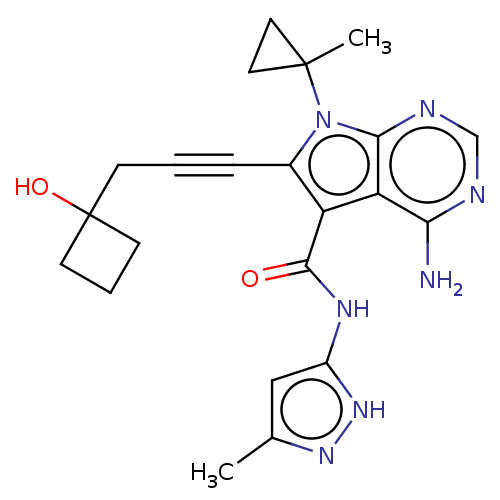
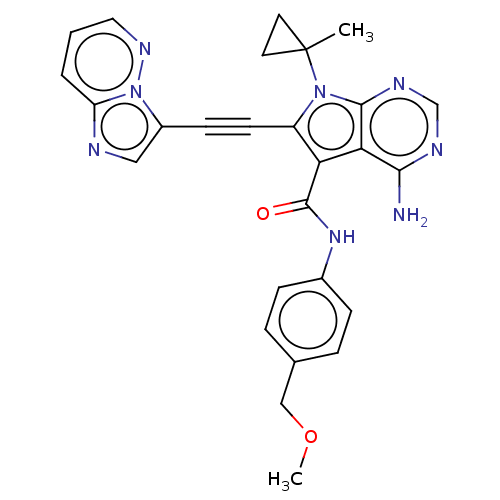
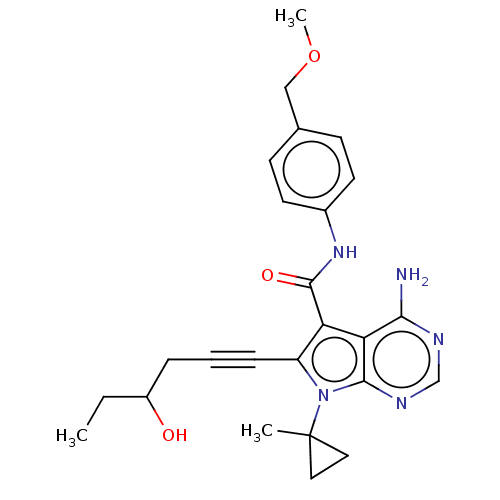
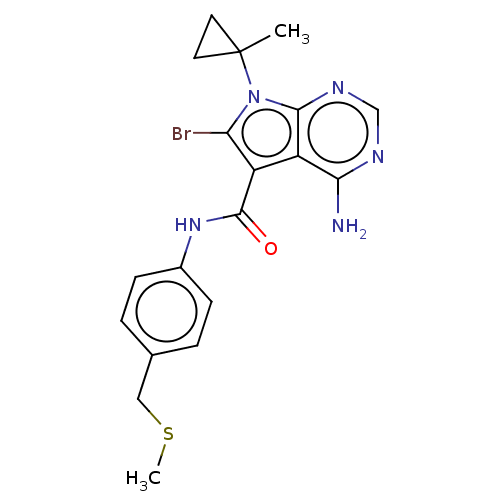
Affinity DataKi: 0.178nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2.09nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 62nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 427nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 537nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 708nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 708nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 794nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.78E+3nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.91E+3nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2.29E+3nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2.40E+3nMAssay Description:Displacement of [3H]histamine from human histamine H4 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2.63E+3nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2.90E+3nMAssay Description:Displacement of [3H]histamine from human histamine H4 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 3.24E+3nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 4.17E+3nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 4.57E+3nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 5.37E+3nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 6.31E+3nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 8.13E+3nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.07E+4nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+4nMAssay Description:Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.0400nMAssay Description:To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.0400nMAssay Description:Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.0400nMAssay Description:To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.0400nMAssay Description:To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.0700nMAssay Description:To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.0700nMAssay Description:Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.0700nMAssay Description:To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.0700nMAssay Description:To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.0900nMAssay Description:To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.0900nMAssay Description:Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.0900nMAssay Description:To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.0900nMAssay Description:To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.100nMAssay Description:Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.100nMAssay Description:Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.100nMAssay Description:Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.100nMAssay Description:Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.100nMAssay Description:To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.100nMAssay Description:To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.100nMAssay Description:To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.100nMAssay Description:Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.100nMAssay Description:Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.100nMAssay Description:Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.100nMAssay Description:To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.100nMAssay Description:Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Taiho Pharmaceutical
US Patent
Taiho Pharmaceutical
US Patent
Affinity DataIC50: 0.100nMAssay Description:Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that...More data for this Ligand-Target Pair