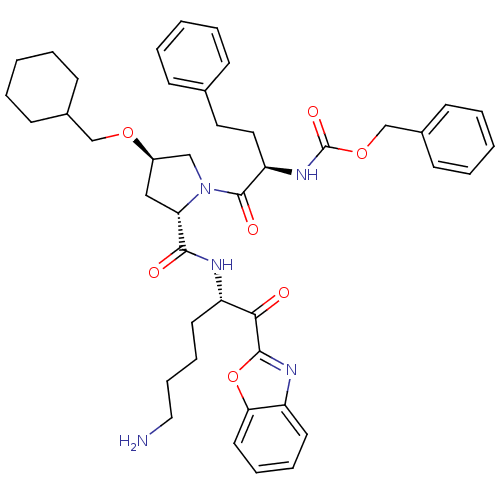
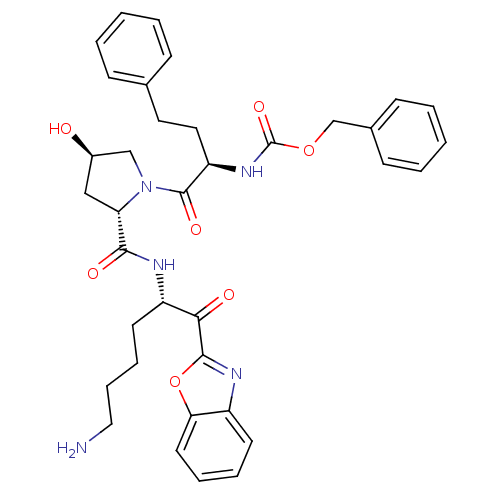
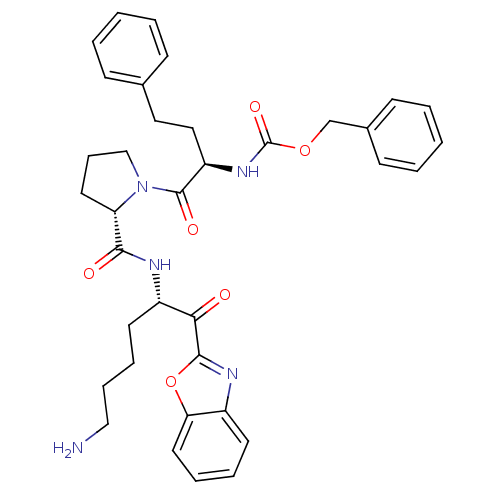
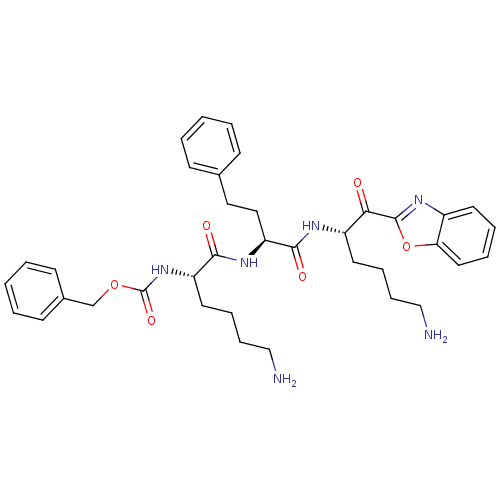
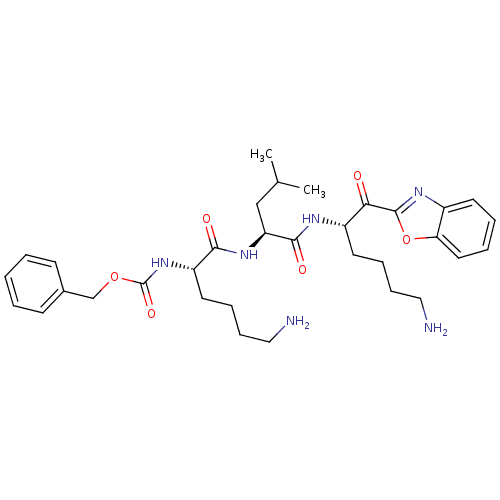
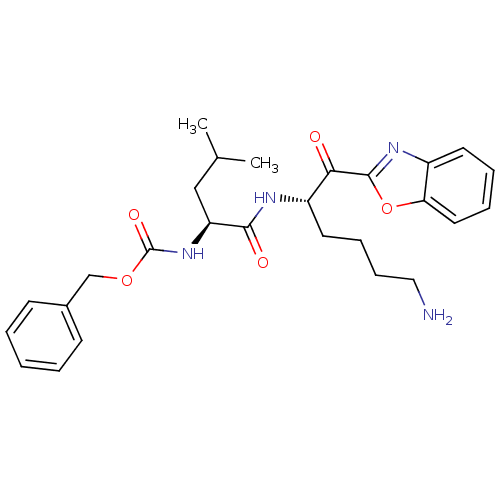
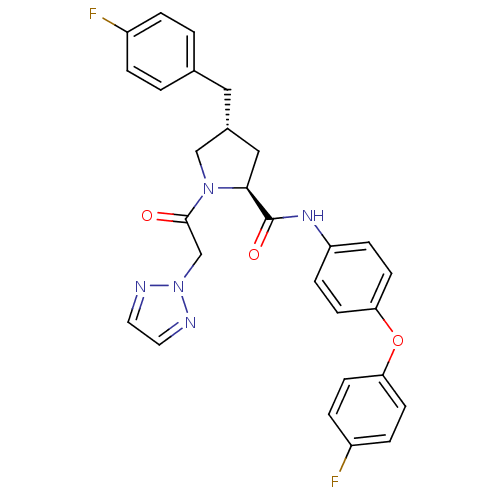
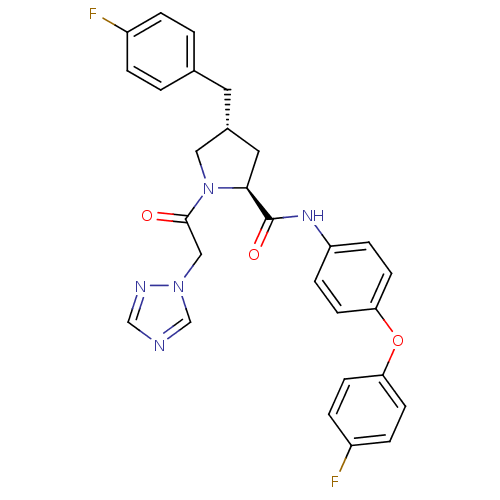
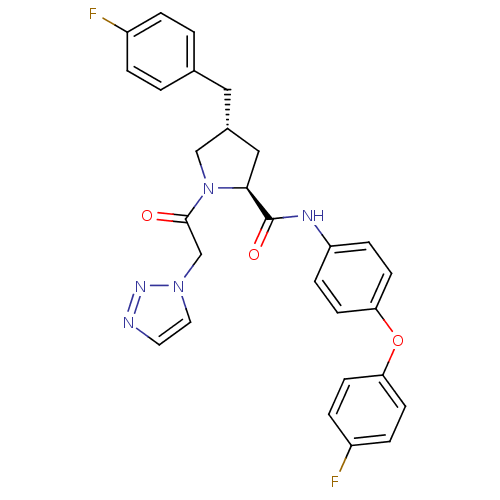
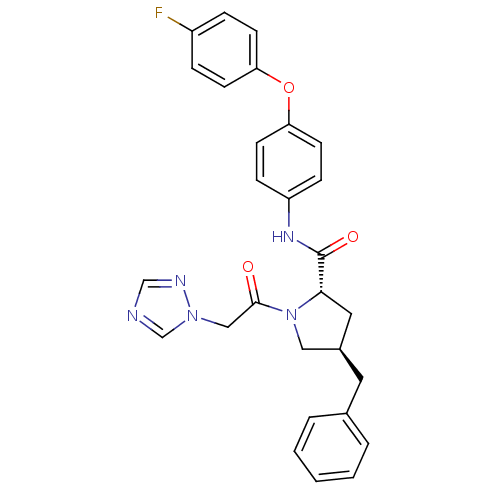
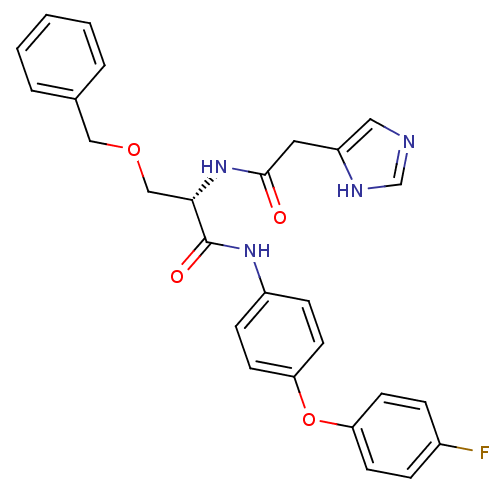
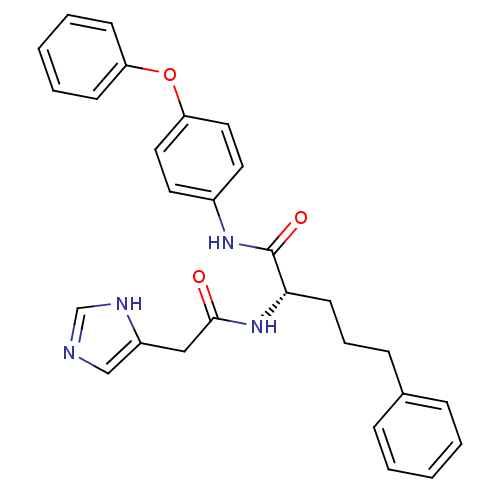
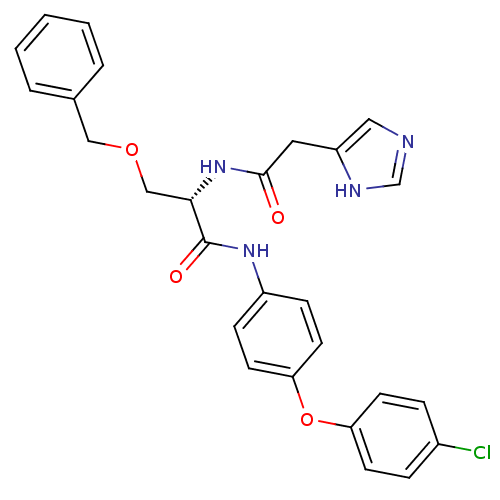
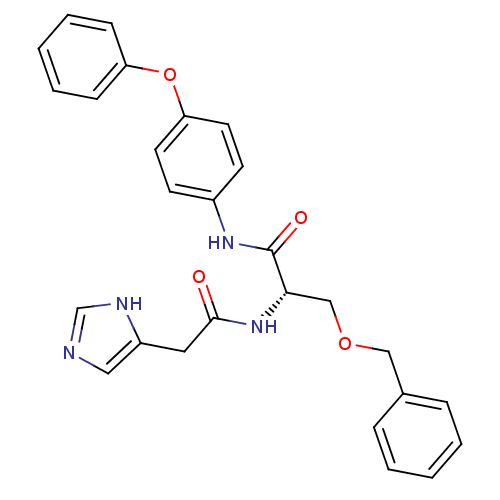
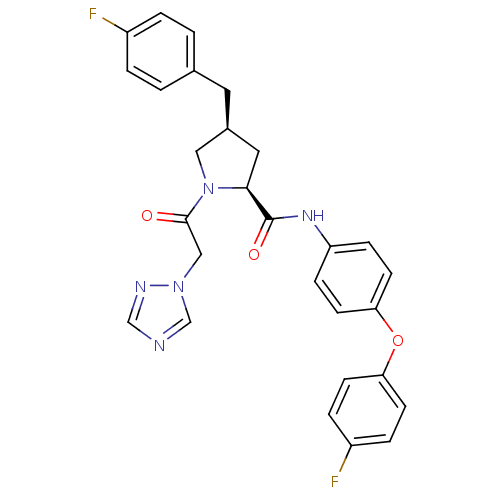
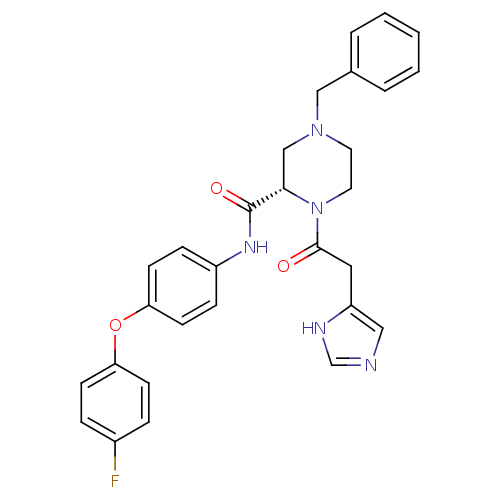
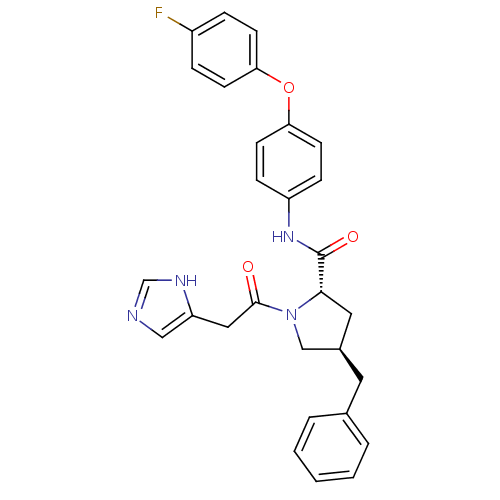
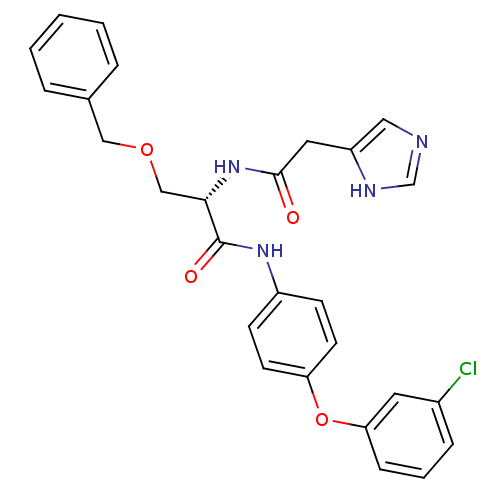
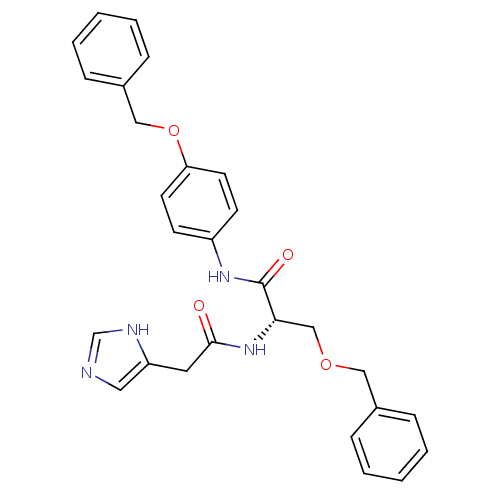
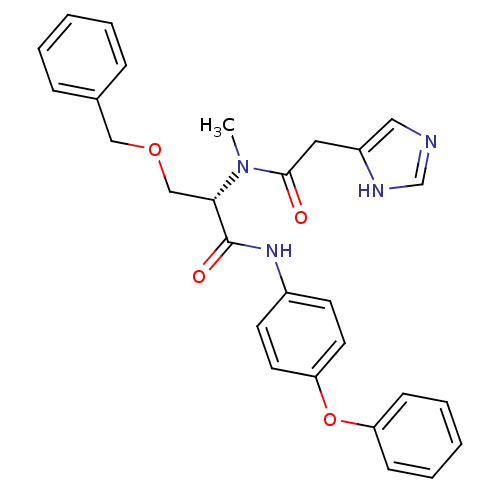
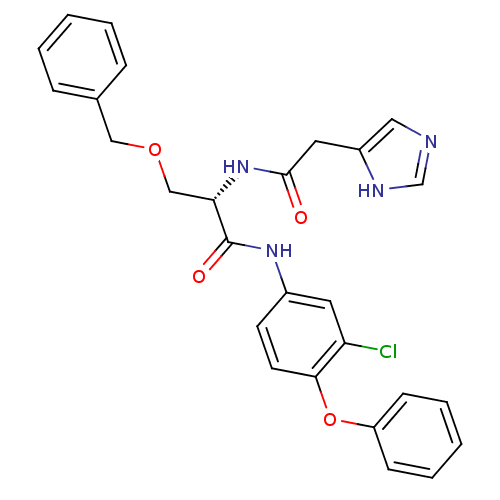
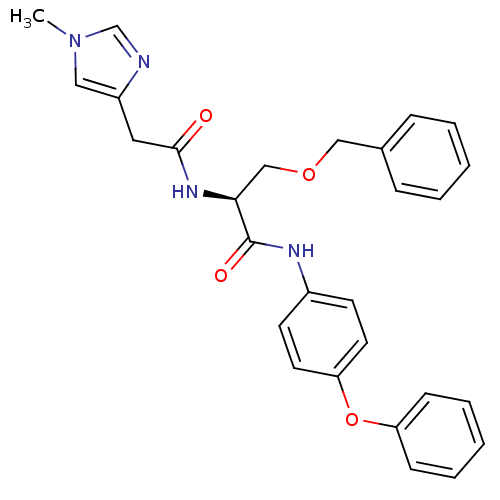
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
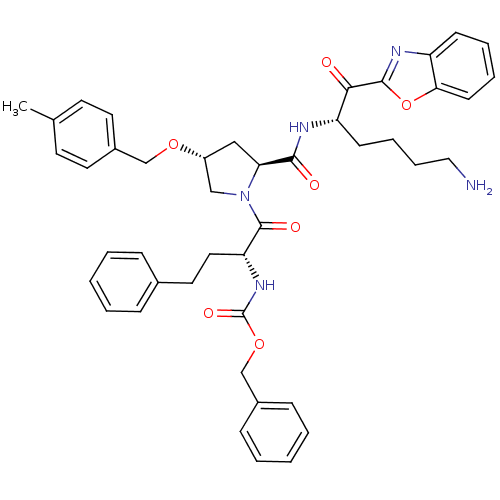
Affinity DataKi: 12nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
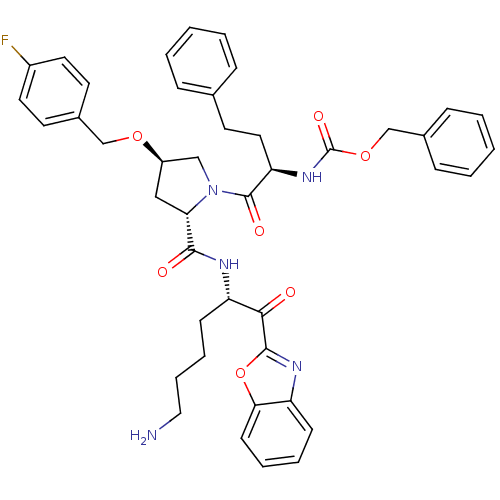
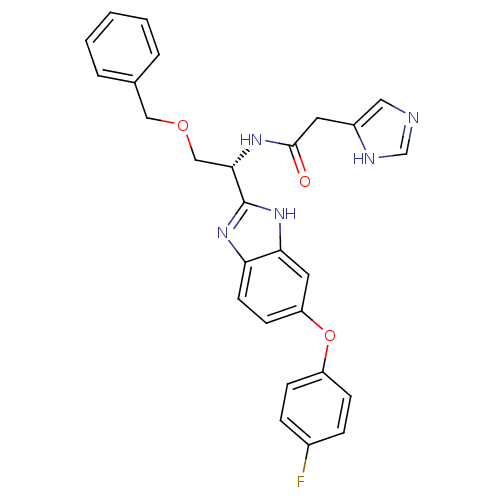
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
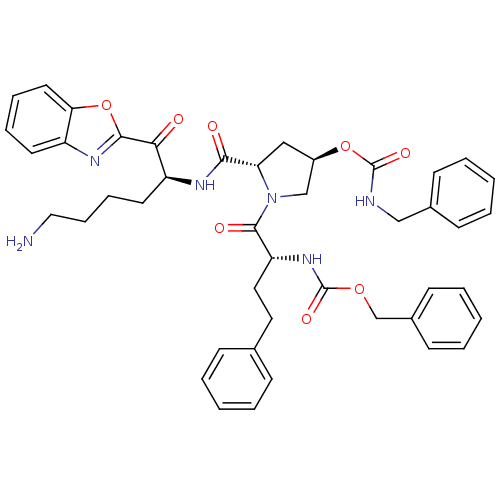
Affinity DataKi: 19nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
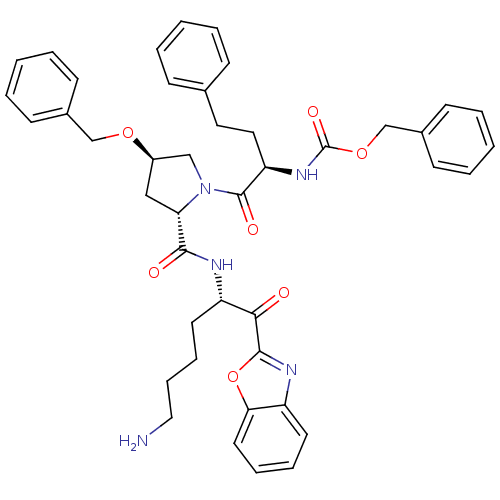
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
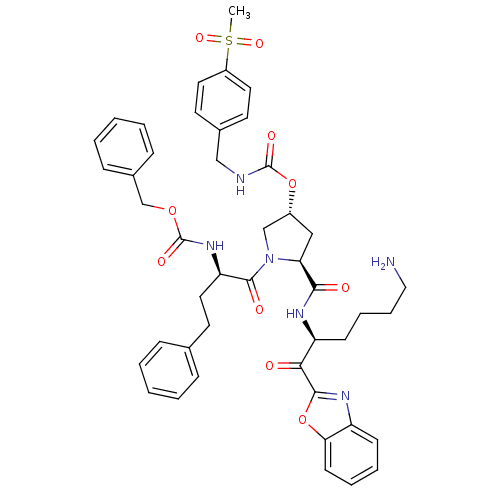
Affinity DataKi: 27nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
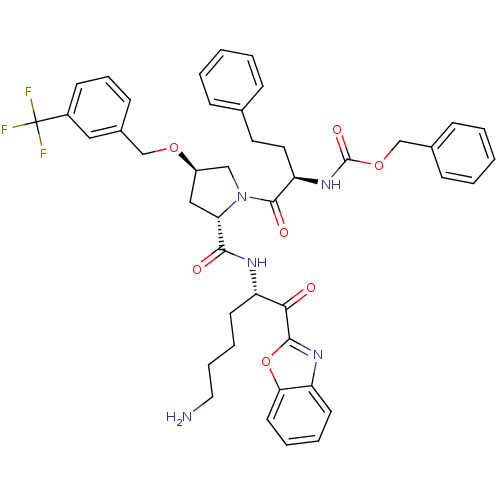
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
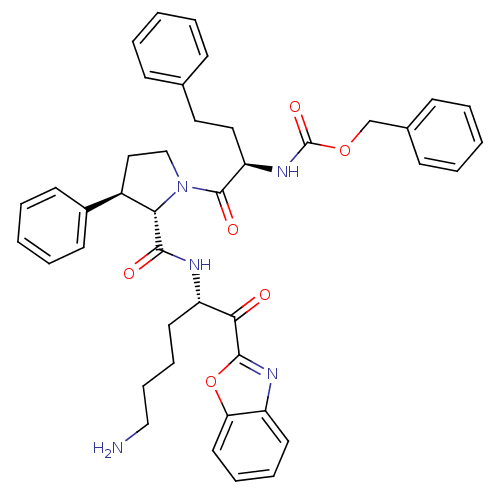
Affinity DataKi: 28nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 29nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 41nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 45nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 49nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 65nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 101nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 130nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 176nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 267nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 510nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 1.04E+3nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 1.40E+3nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 1.55E+3nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 2.10E+3nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 5.72E+3nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 5.78E+3nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 1.52E+4nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 5.80nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 5.80nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 6.80nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced tango beta-arrestin recruitment preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced tango beta-arrestin recruitment preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced tango beta-arrestin recruitment preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 33nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced tango beta-arrestin recruitment preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 48nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 84nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 84.7nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 96nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 152nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 158nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 181nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 211nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 213nMAssay Description:Inhibition of human CYP2C9 using tolbutamide as substrate by LC/MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 221nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced tango beta-arrestin recruitment preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 228nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 239nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 284nMAssay Description:Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced tango beta-arrestin recruitment preincubated for...More data for this Ligand-Target Pair