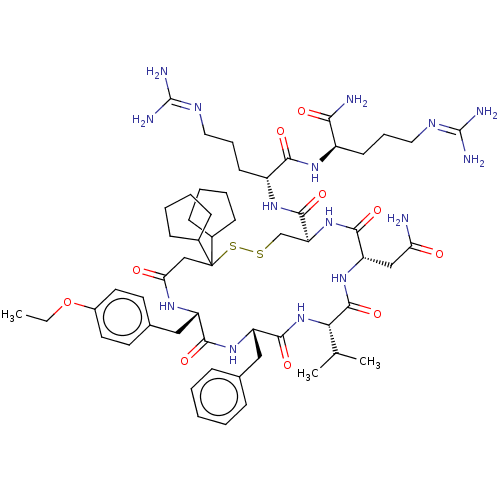
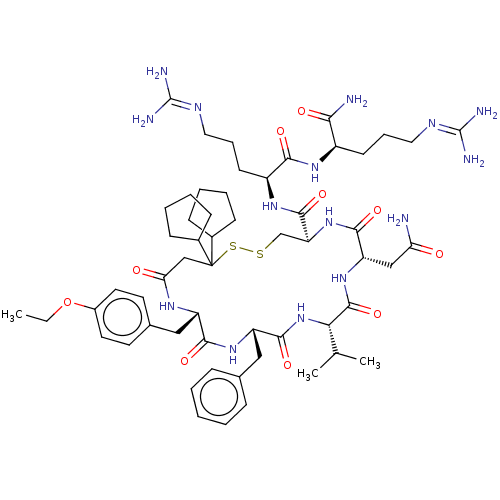
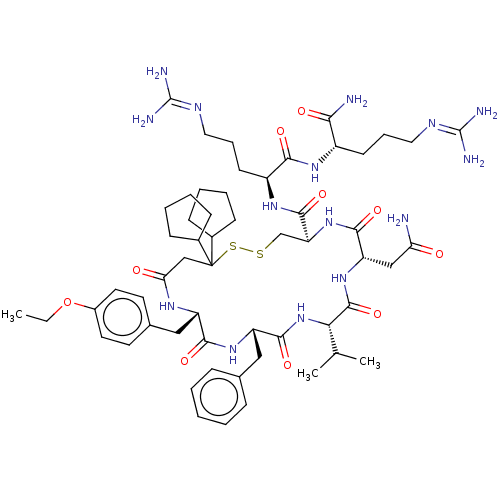
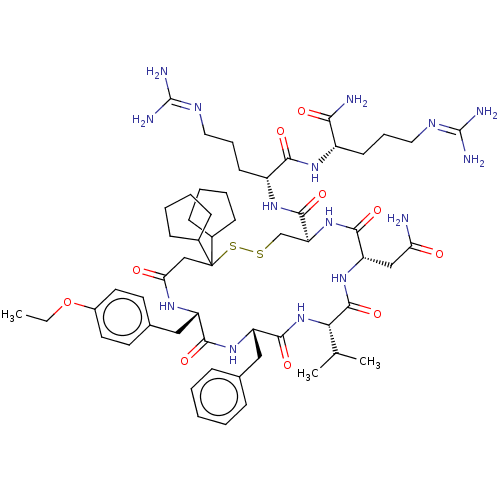
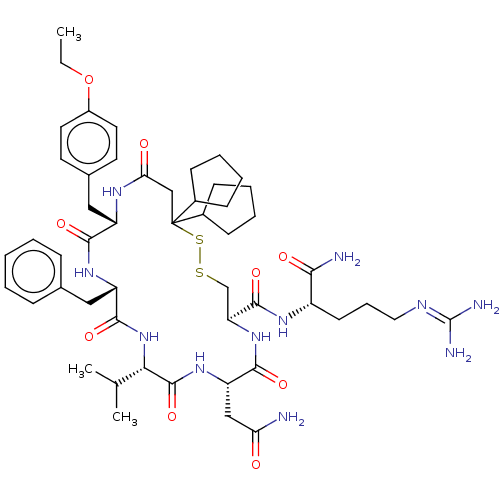
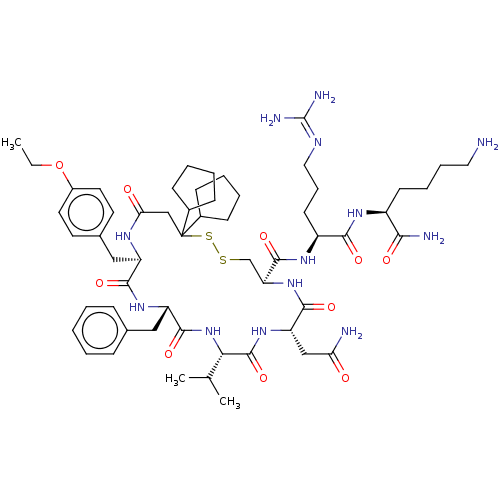
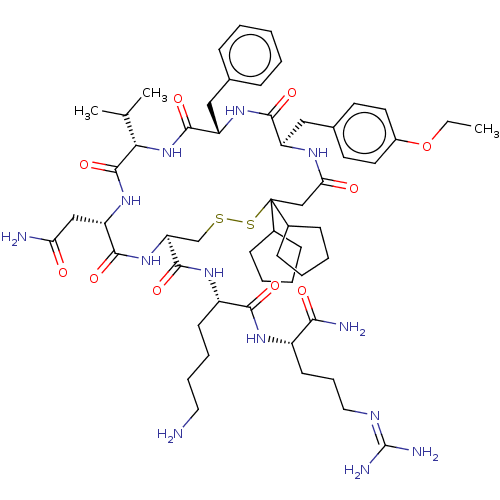
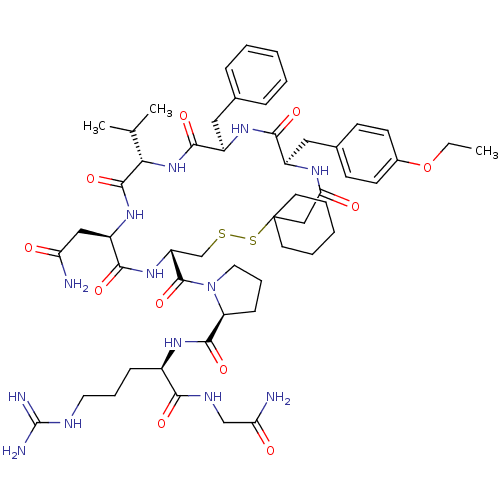
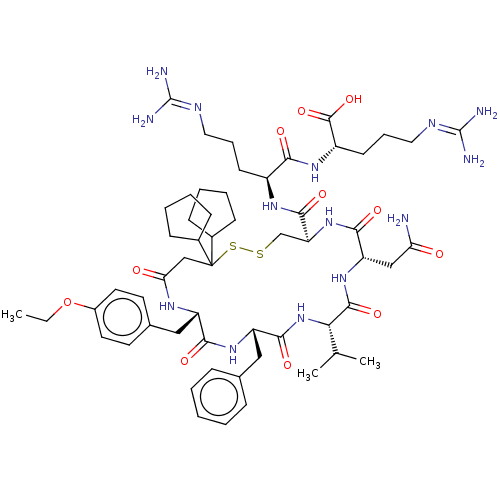
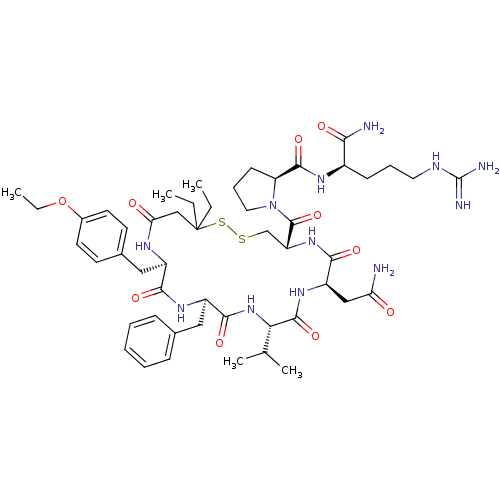
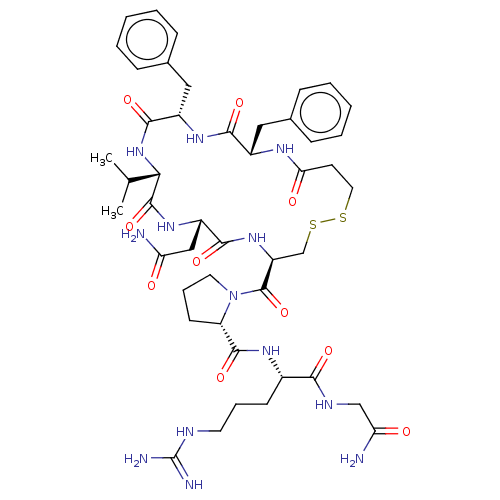
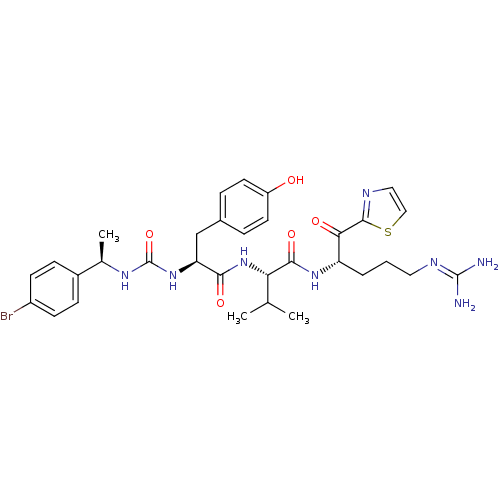
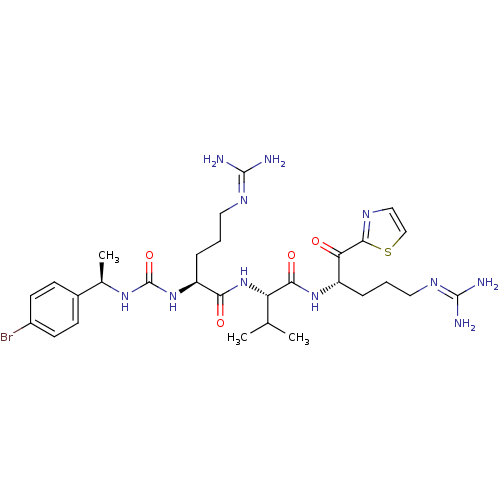
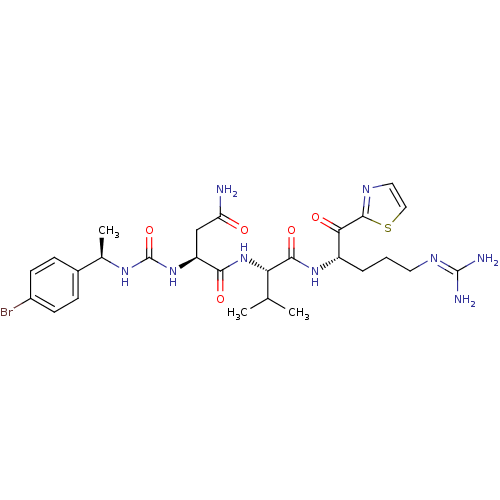
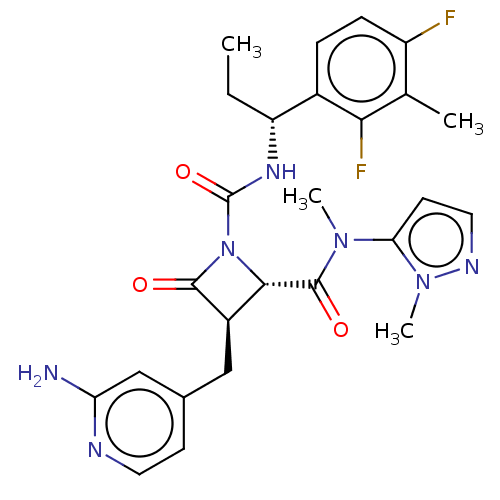
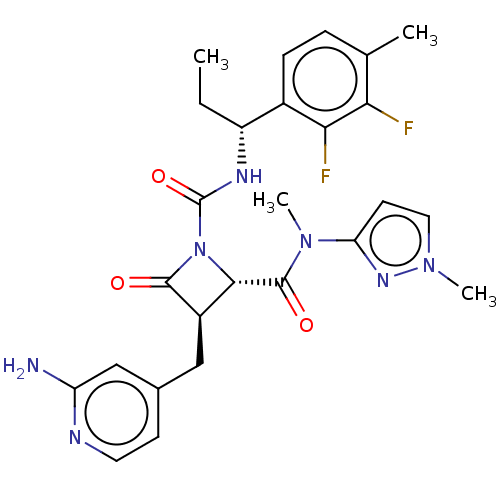
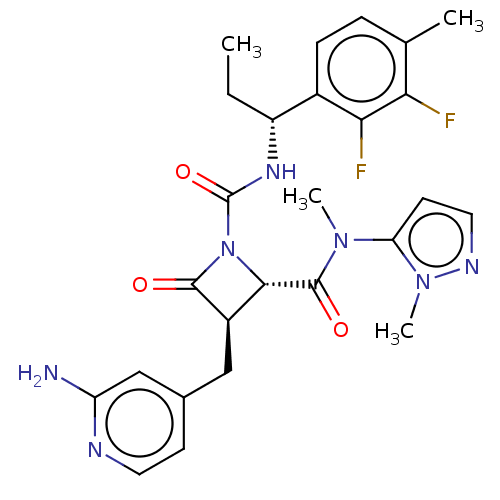
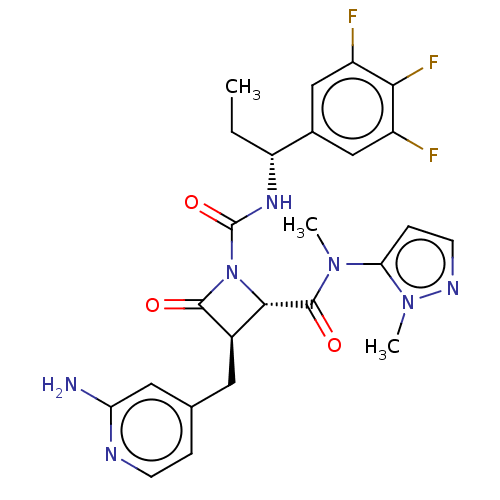
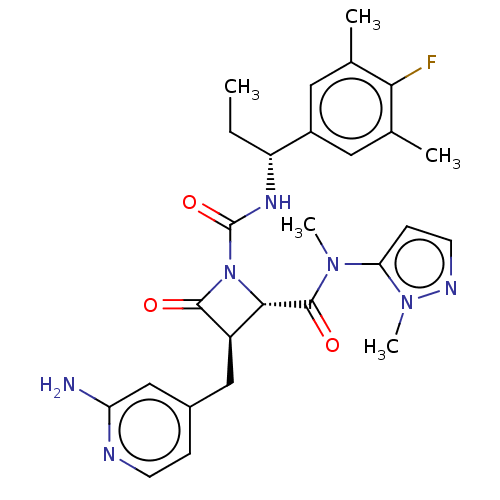
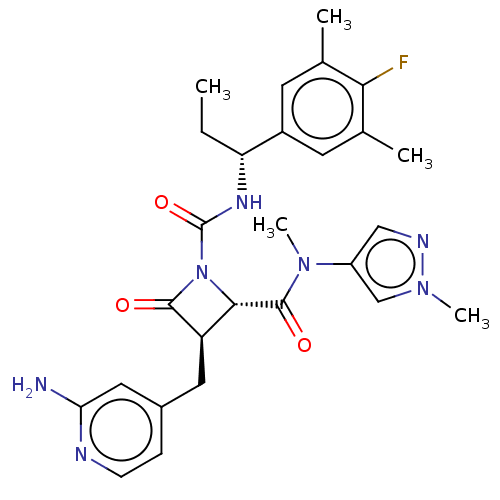
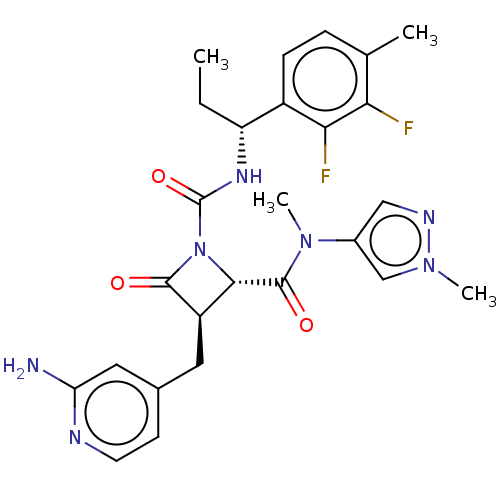
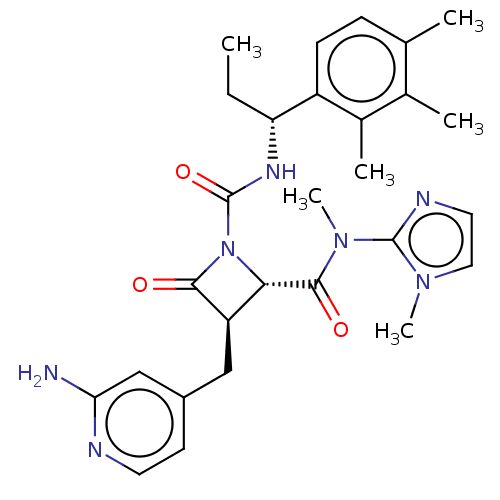
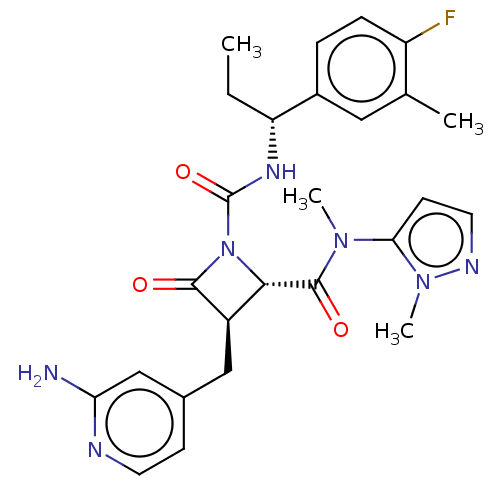
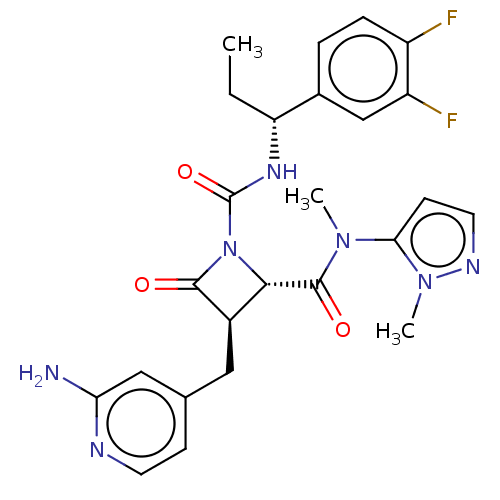
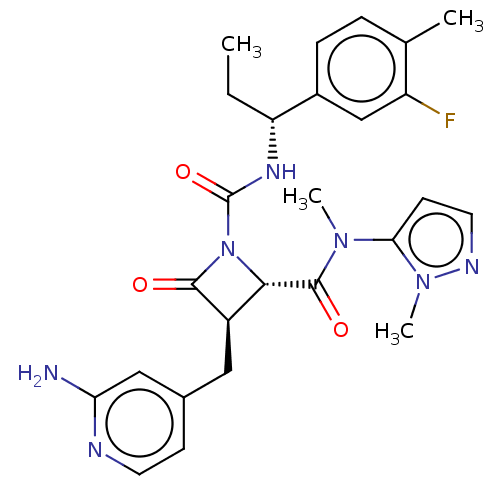
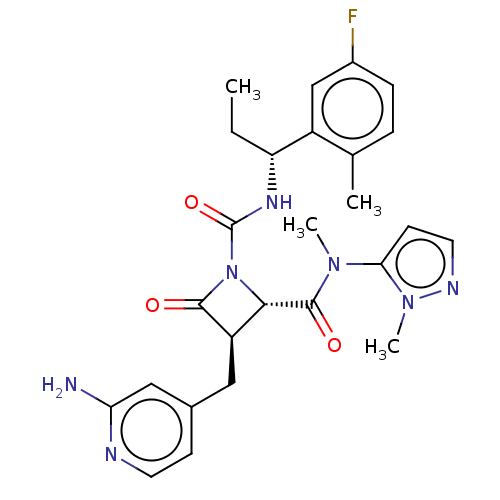
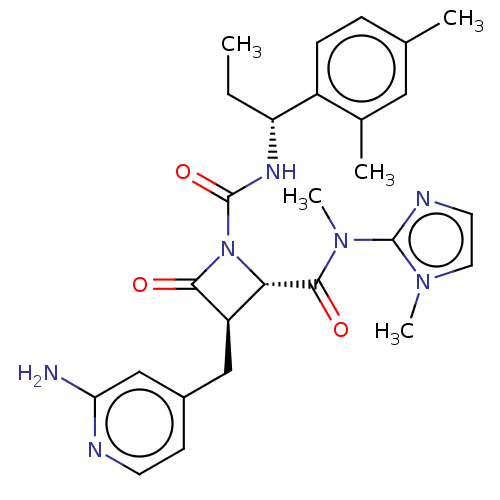
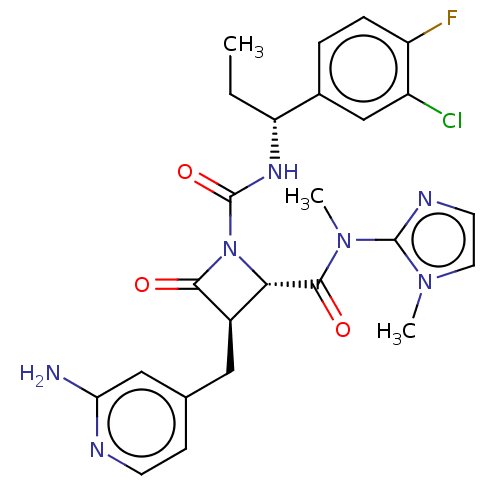
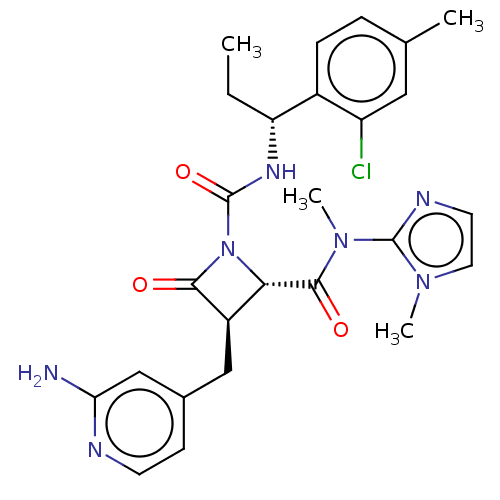
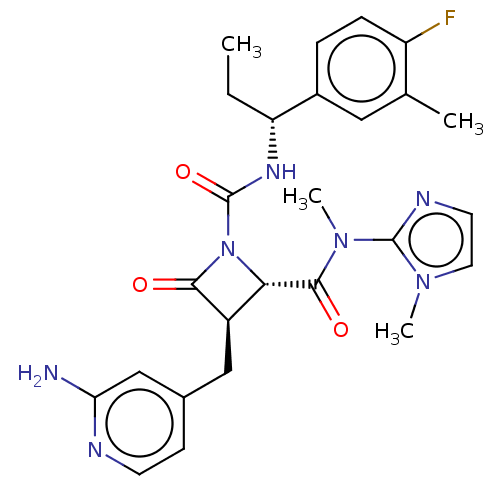
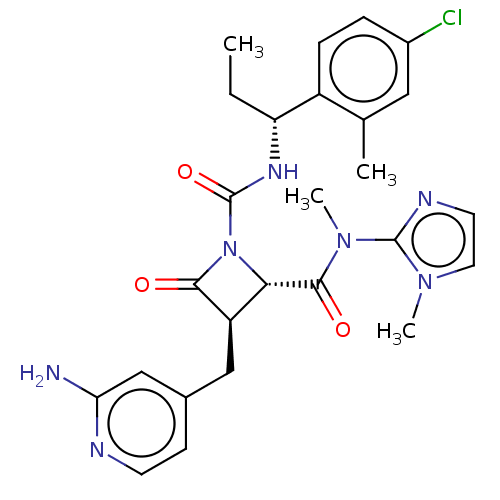
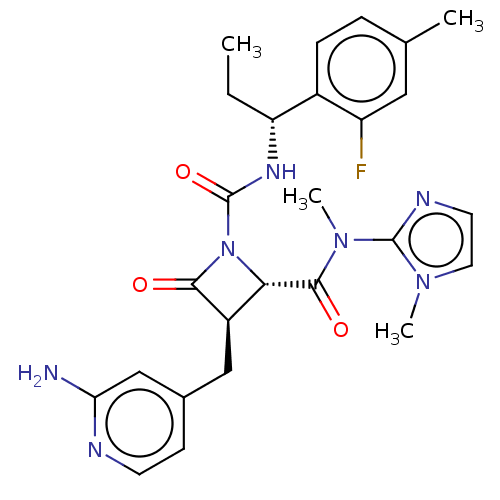
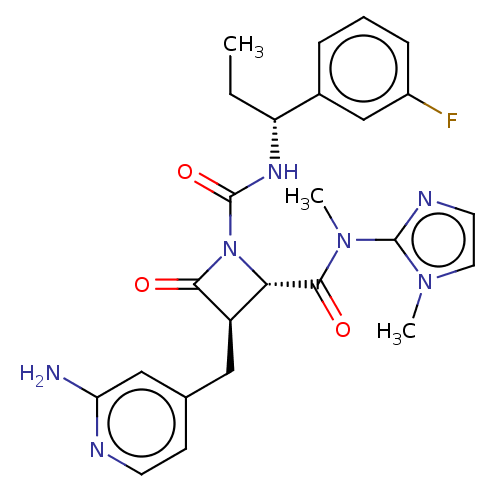
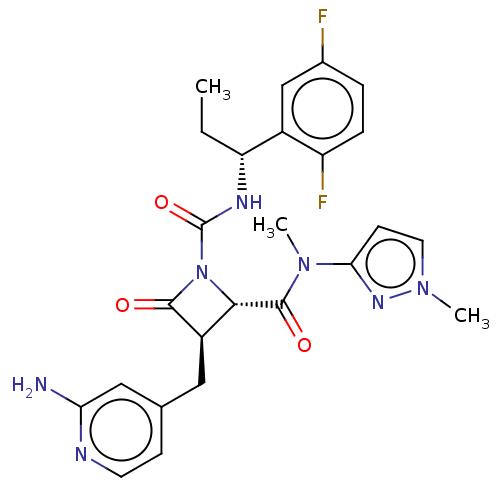
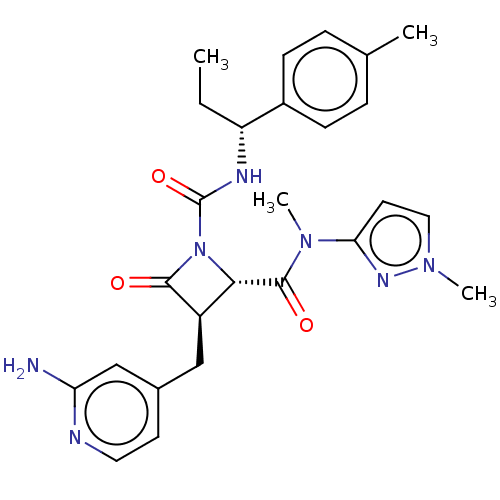
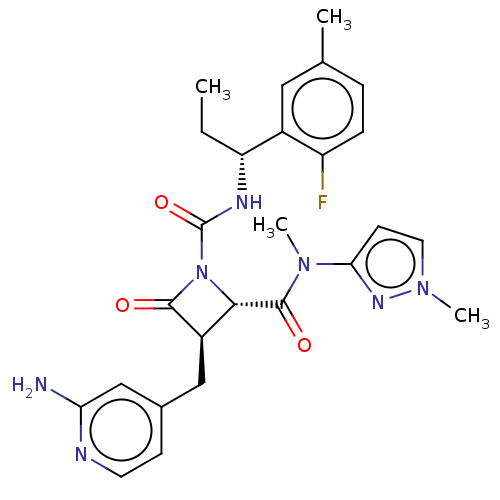
TargetAdenylate cyclase type 4(Homo sapiens (Human))
Research And Development Division
Curated by ChEMBL
Research And Development Division
Curated by ChEMBL
Affinity DataKi: 0.880nMAssay Description:Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperoneMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 4(Homo sapiens (Human))
Research And Development Division
Curated by ChEMBL
Research And Development Division
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperoneMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 4(Homo sapiens (Human))
Research And Development Division
Curated by ChEMBL
Research And Development Division
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperoneMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 4(Homo sapiens (Human))
Research And Development Division
Curated by ChEMBL
Research And Development Division
Curated by ChEMBL
Affinity DataKi: 1.90nMAssay Description:Binding affinity against sigma receptorMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 4(Homo sapiens (Human))
Research And Development Division
Curated by ChEMBL
Research And Development Division
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperoneMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 4(Homo sapiens (Human))
Research And Development Division
Curated by ChEMBL
Research And Development Division
Curated by ChEMBL
Affinity DataKi: 2.60nMAssay Description:Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperoneMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 4(Homo sapiens (Human))
Research And Development Division
Curated by ChEMBL
Research And Development Division
Curated by ChEMBL
Affinity DataKi: 3.90nMAssay Description:Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperoneMore data for this Ligand-Target Pair
Affinity DataKi: 3.90nMAssay Description:Compound was tested for inhibition against V2 vasopressin receptor in pig renal medullary membrane preparations.More data for this Ligand-Target Pair
TargetAdenylate cyclase type 4(Homo sapiens (Human))
Research And Development Division
Curated by ChEMBL
Research And Development Division
Curated by ChEMBL
Affinity DataKi: 4.20nMAssay Description:Binding affinity against sigma receptorMore data for this Ligand-Target Pair
Affinity DataKi: 5.30nMAssay Description:Compound was tested for inhibition against V2 vasopressin receptor in pig renal medullary membrane preparations.More data for this Ligand-Target Pair
Affinity DataKi: 6.40nMAssay Description:Compound was tested for inhibition against V2 vasopressin receptor in pig renal medullary membrane preparations.More data for this Ligand-Target Pair
TargetAdenylate cyclase type 4(Homo sapiens (Human))
Research And Development Division
Curated by ChEMBL
Research And Development Division
Curated by ChEMBL
Affinity DataKi: 7.80nMAssay Description:Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperoneMore data for this Ligand-Target Pair
Affinity DataKi: 8.90nMAssay Description:Compound was tested for inhibition against V2 vasopressin receptor in pig renal medullary membrane preparations.More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Inhibition of LVP-sensitive adenylate cyclase in a pig renal medullary preparationMore data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:Inhibition of LVP-sensitive adenylate cyclase in a pig renal medullary preparationMore data for this Ligand-Target Pair
Affinity DataKi: 7.00E+3nMAssay Description:Inhibition of LVP-sensitive adenylate cyclase in a pig renal medullary preparationMore data for this Ligand-Target Pair
Affinity DataKi: 9.20E+3nMAssay Description:Inhibition of LVP-sensitive adenylate cyclase in a pig renal medullary preparationMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The ability of compounds of the present invention to inhibit Factor XIa was evaluated by determining the concentration of inhibitor, which resulted i...More data for this Ligand-Target Pair