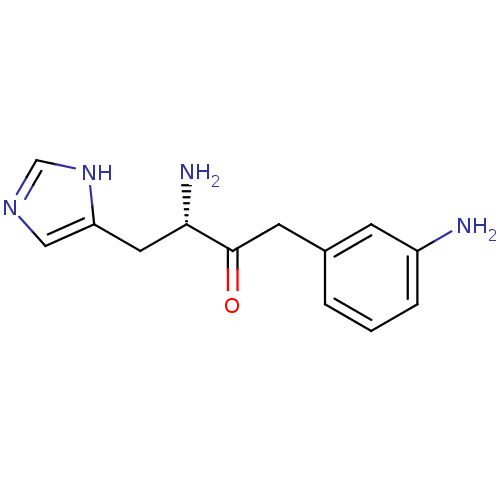
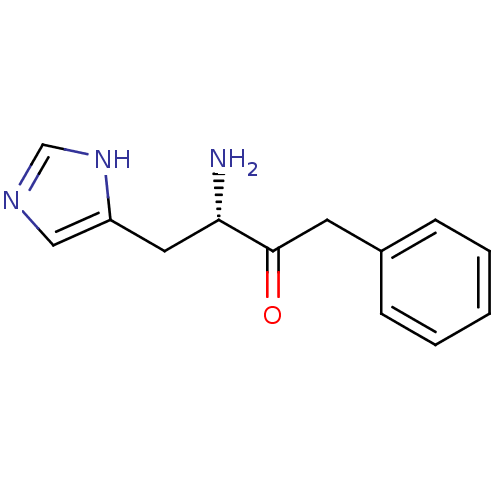
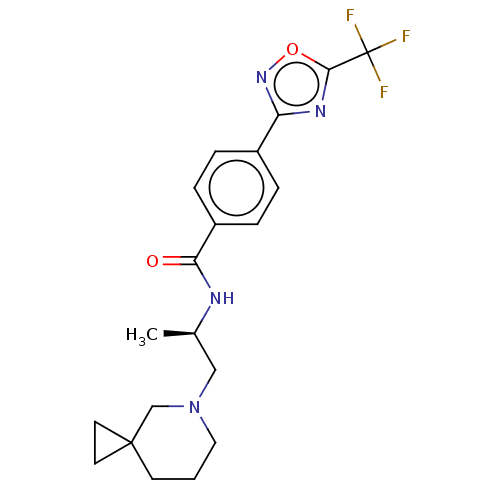
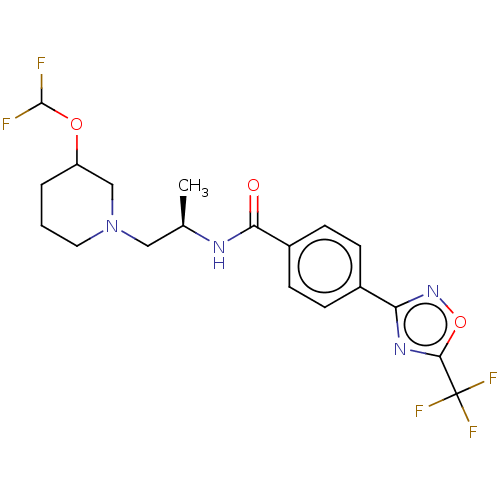
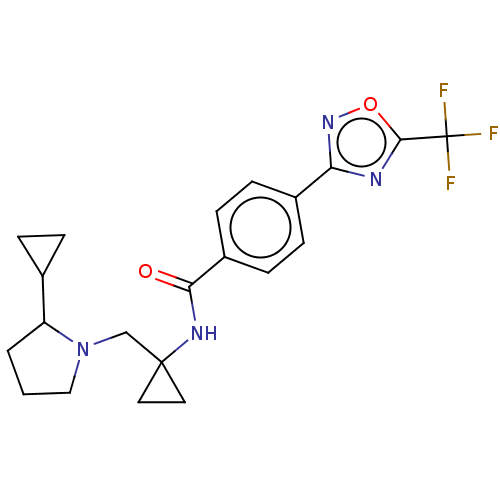
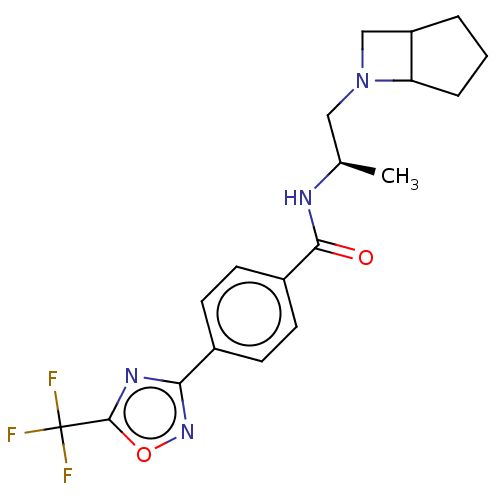
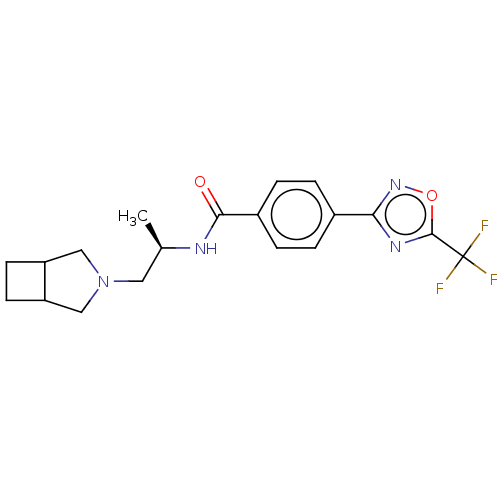
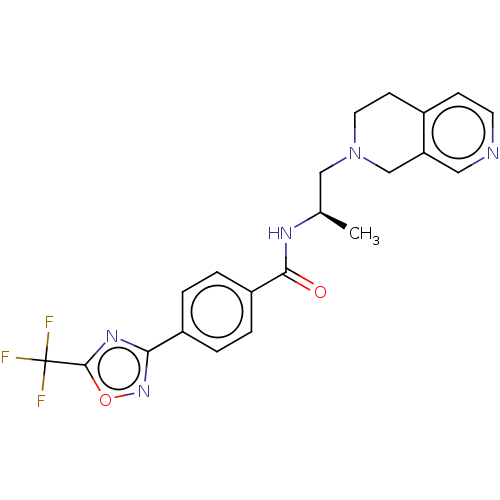
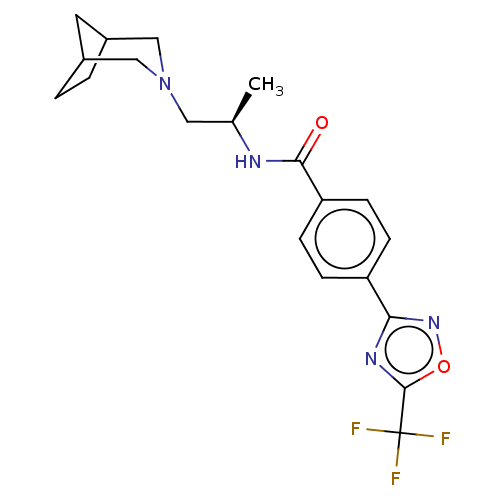
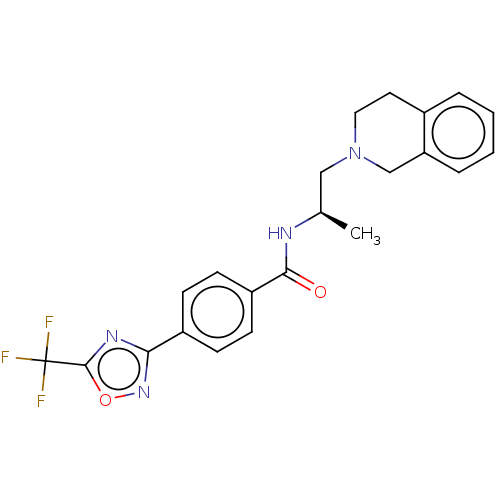
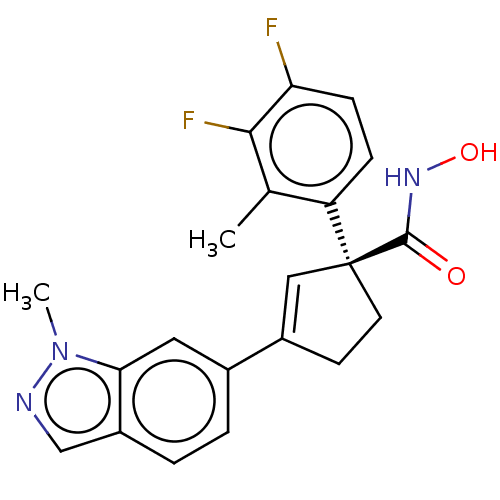
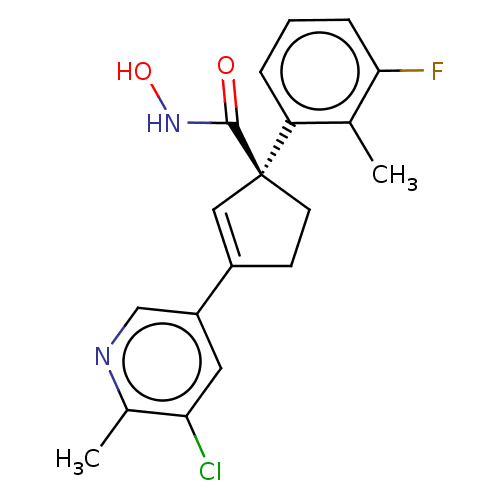
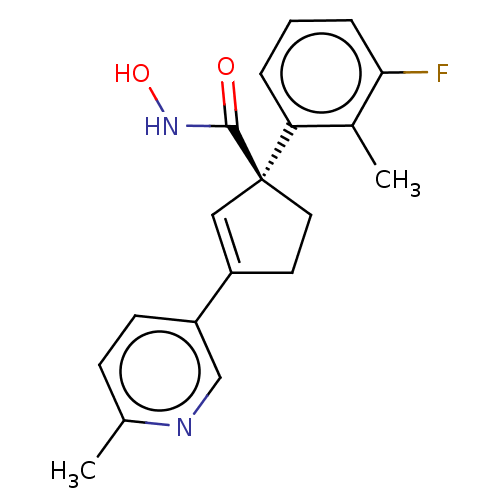
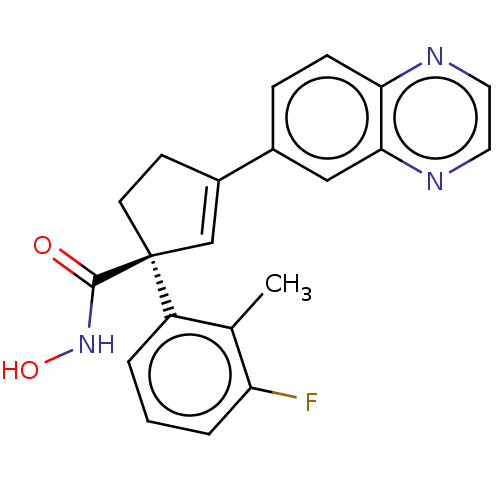
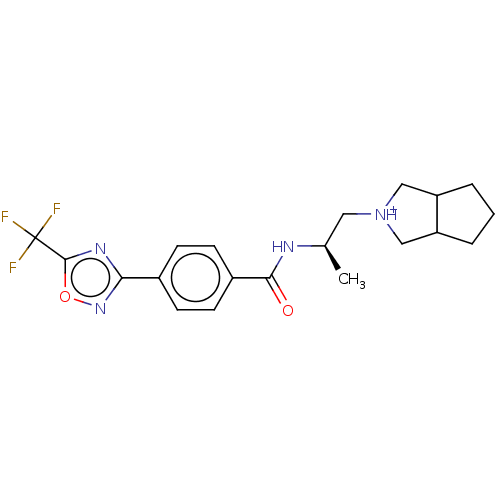
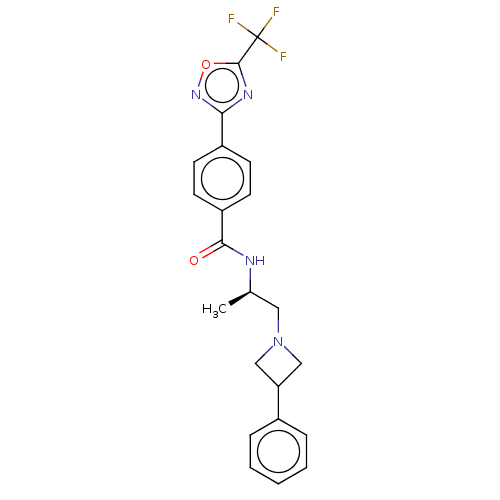
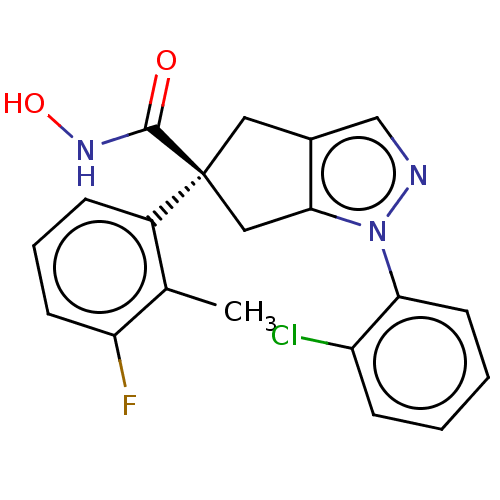
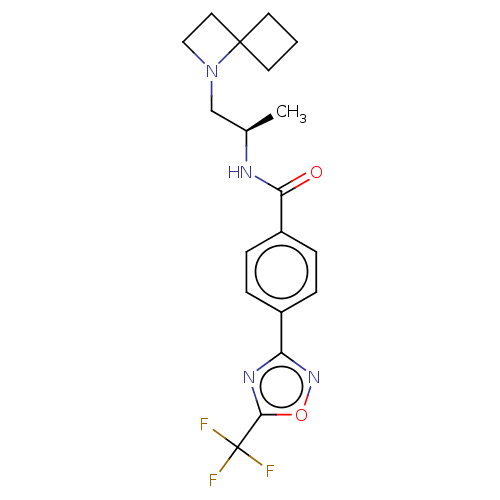
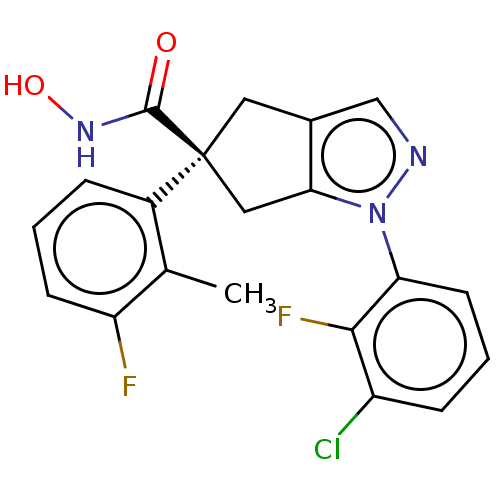
Affinity DataKi: 2.90nMAssay Description:Inhibition of Brassica oleracea (cabbage) histidinol dehydrogenase pre-incubated for 10 min before substrate histidinol addition by spectrophotometryMore data for this Ligand-Target Pair
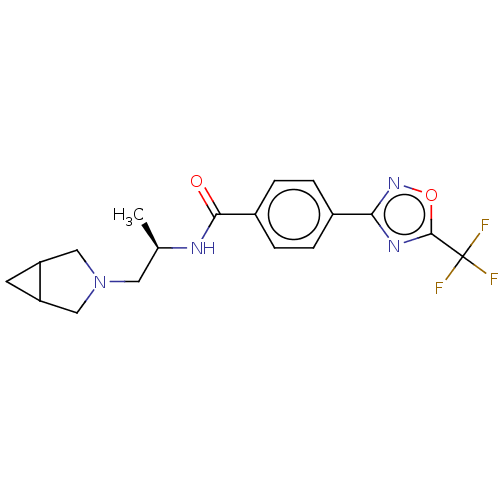
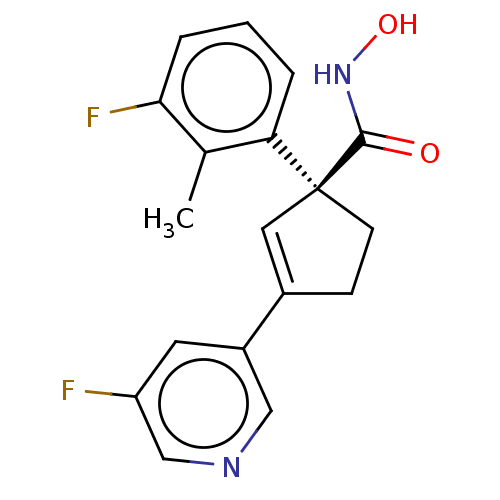
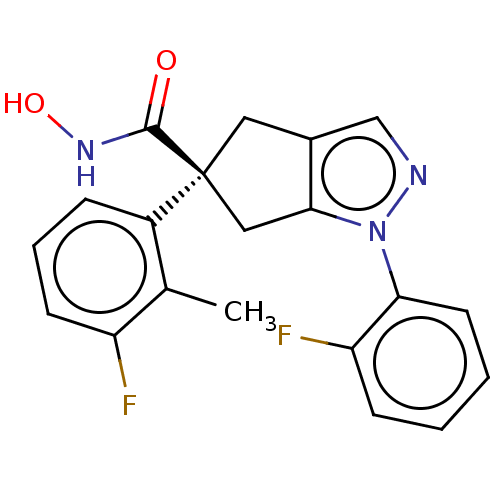
Affinity DataKi: 4.40nMAssay Description:Inhibition of Brassica oleracea (cabbage) histidinol dehydrogenase pre-incubated for 10 min before substrate histidinol addition by spectrophotometryMore data for this Ligand-Target Pair
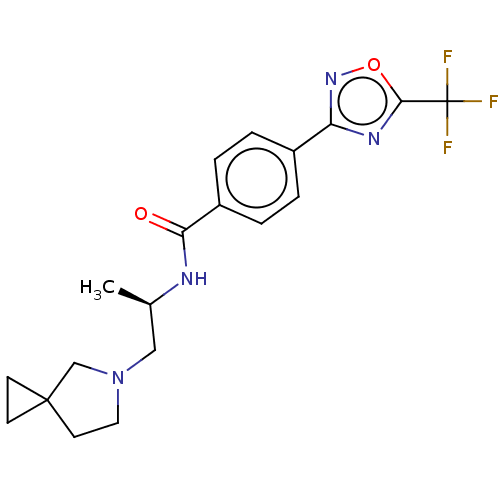
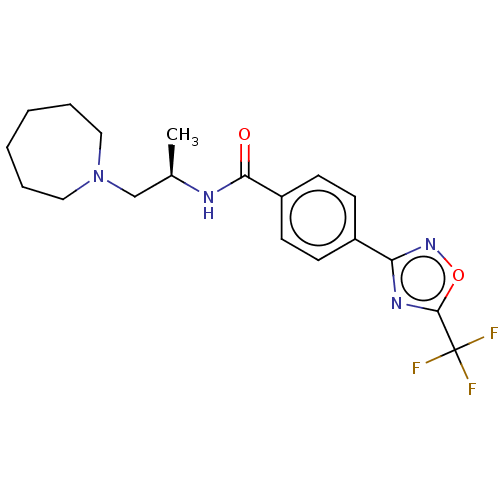
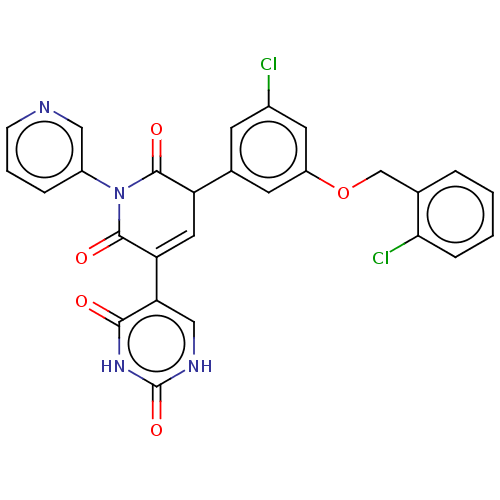
Affinity DataKi: 7.40nMAssay Description:Inhibition of Brassica oleracea (cabbage) histidinol dehydrogenase pre-incubated for 10 min before substrate histidinol addition by spectrophotometryMore data for this Ligand-Target Pair
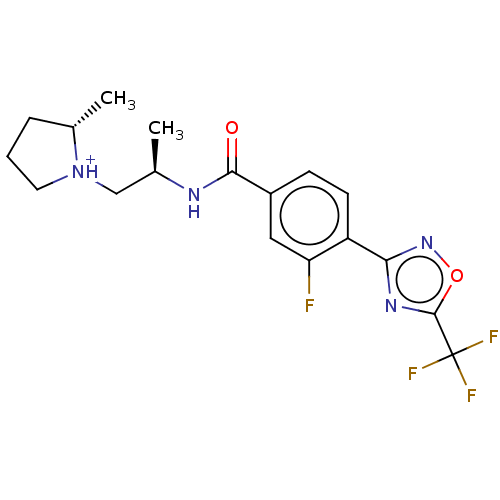
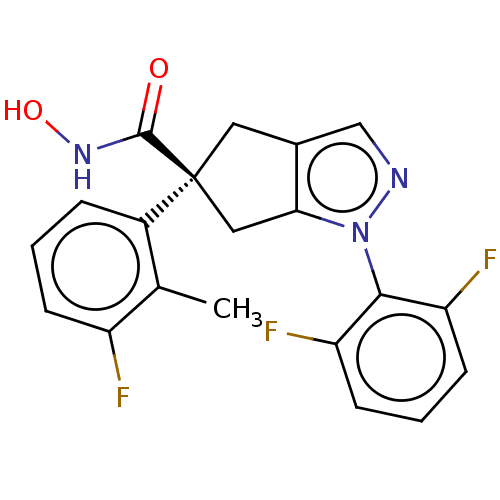
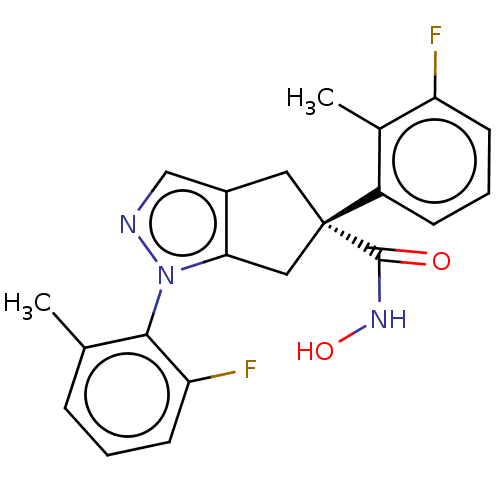
Affinity DataKi: 7.60nMAssay Description:Inhibition of Brassica oleracea (cabbage) histidinol dehydrogenase pre-incubated for 10 min before substrate histidinol addition by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:The Class I HDAC activity of Class IIa Histone Deacetylase (HDAC) inhibitors was quantified by measuring the cellular histone deacetylase enzymatic a...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:The Class I HDAC activity of Class IIa Histone Deacetylase (HDAC) inhibitors was quantified by measuring the cellular histone deacetylase enzymatic a...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibition of proteolytic activity was tested using recombinant SARS-CoV-2Mpro, which was expressed and purified as previously described.8,12 For the...More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair