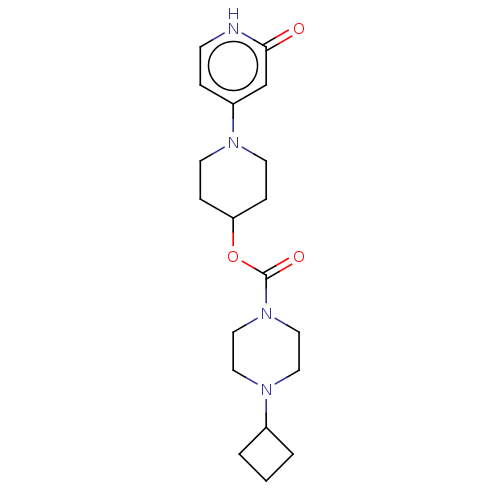
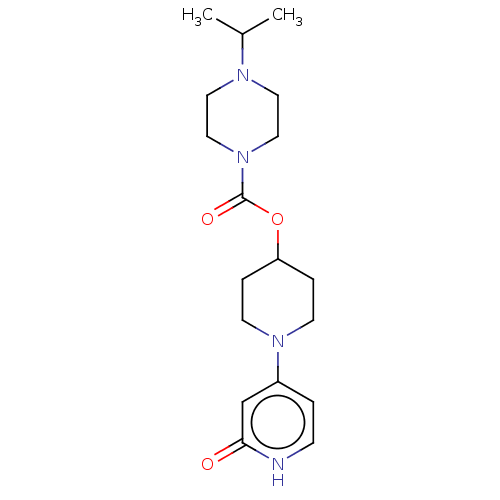
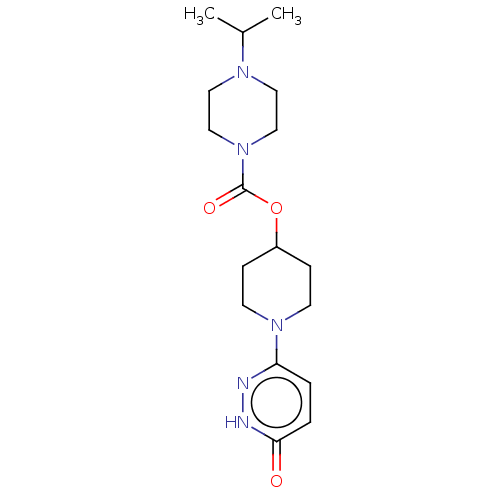
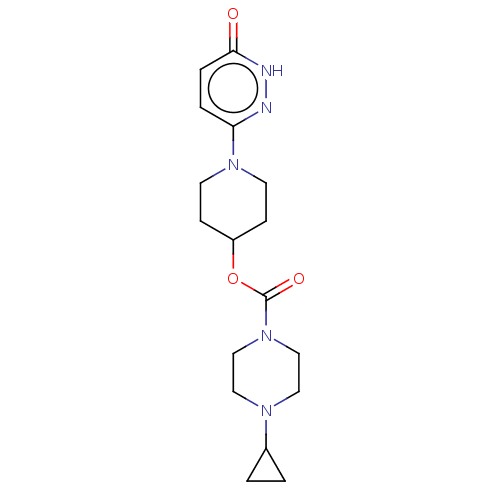
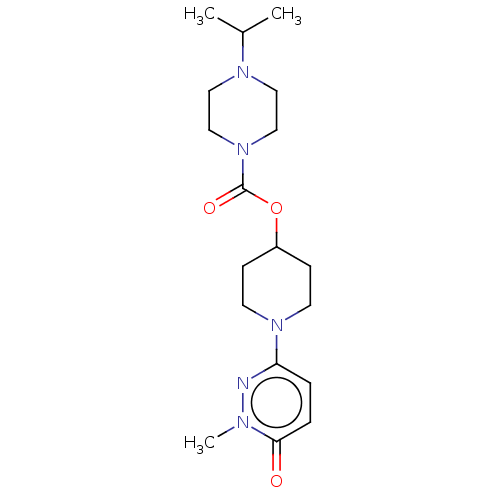
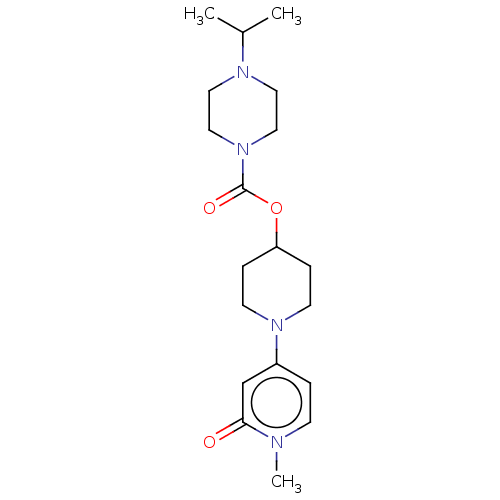
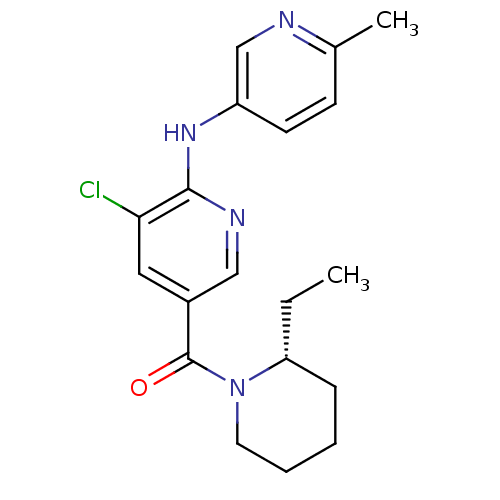
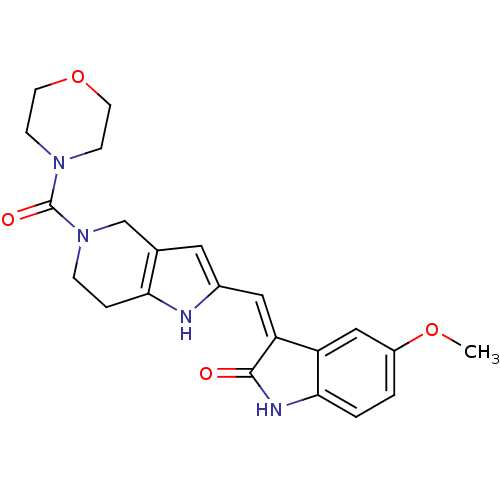
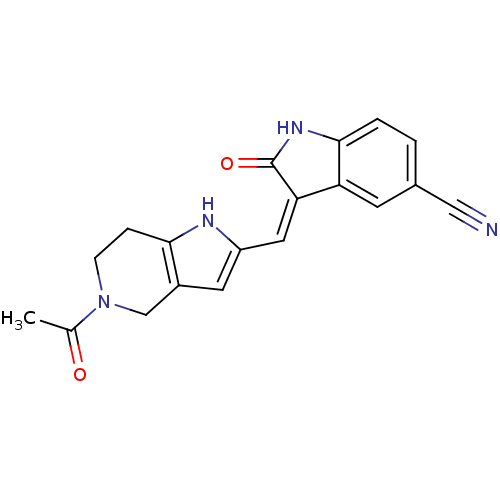
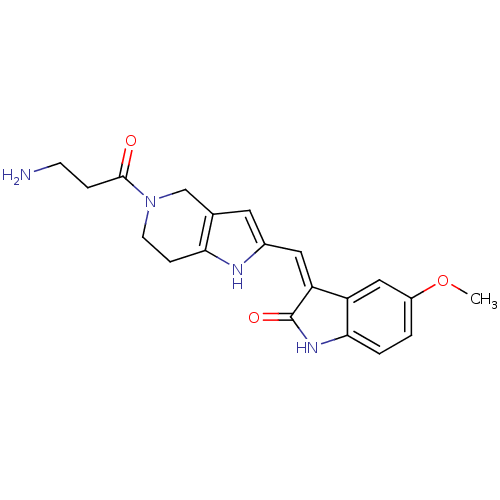
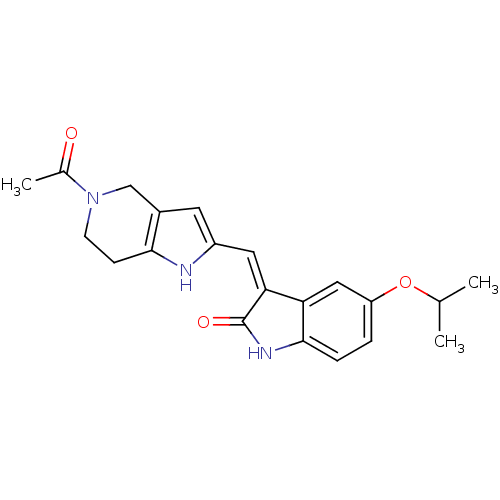
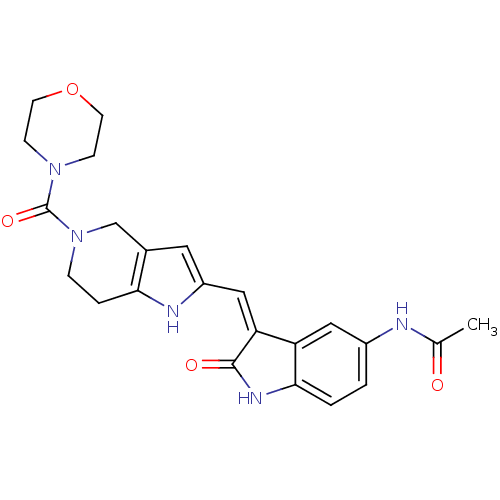
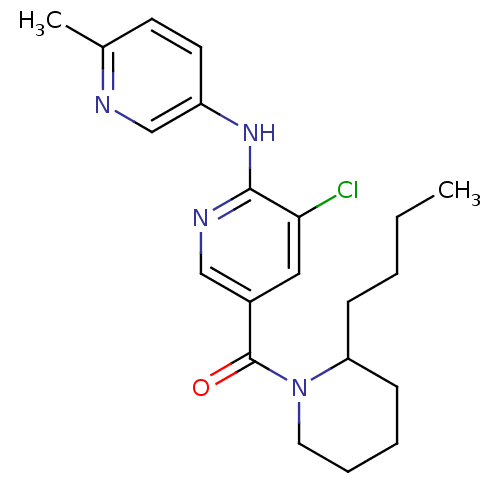
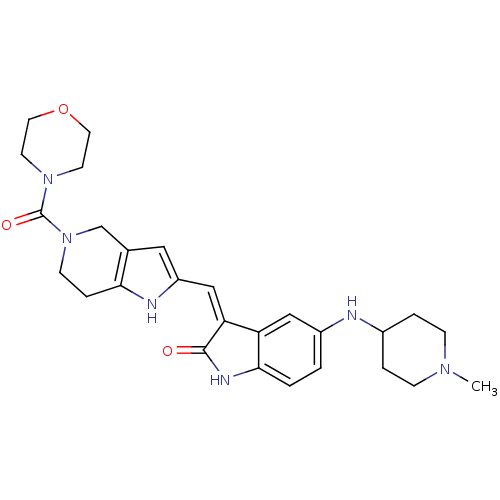
Affinity DataKi: 0.5nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
Affinity DataKi: 2.40nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
Affinity DataKi: 2.90nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
Affinity DataKi: 3.10nMAssay Description:The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ...More data for this Ligand-Target Pair
Affinity DataKi: 10nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
Affinity DataKi: 12nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
Affinity DataKi: 20nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
Affinity DataKi: 25nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
Affinity DataKi: 25nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
Affinity DataKi: 25nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
Affinity DataKi: 26nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Rattus norvegicus (Rat))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 30nMAssay Description:Displacement of [3H]ABP688 from mGluR5 in rat brain cortexMore data for this Ligand-Target Pair
Affinity DataKi: 31nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 38nMAssay Description:Displacement of [3H]ABP688 from human recombinant mGluR5 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 44nMpH: 7.5Assay Description:The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human LRRK2 (1885 to 2132) using 5-Fluo-Ahx-RLGRDKYKTLRQIRQGNTK-OH as substrate after 60 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of RET (unknown origin)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 8(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human COT (66 to 395 residues) expressed in Sf21 cells using 5-Fluo-Ahx-AGAGSGQLIDSNleANSFVGTR-NH2 as substrate after 60 mins by calipe...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of RET (unknown origin)More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human LRRK2 (1885 to 2132) using 5-Fluo-Ahx-RLGRDKYKTLRQIRQGNTK-OH as substrate after 60 mins by fluorescence assayMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human LRRK2 (1885 to 2132) using 5-Fluo-Ahx-RLGRDKYKTLRQIRQGNTK-OH as substrate after 60 mins by fluorescence assayMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Antagonist activity at human mGluR5 assessed as inhibition of quisqualate-induced intracellular inositol phosphate accumulationMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of RET (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 8(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of human COT (66 to 395 residues) expressed in Sf21 cells using 5-Fluo-Ahx-AGAGSGQLIDSNleANSFVGTR-NH2 as substrate after 60 mins by calipe...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair