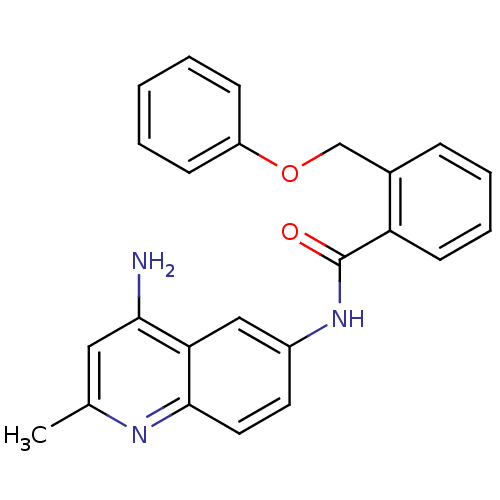
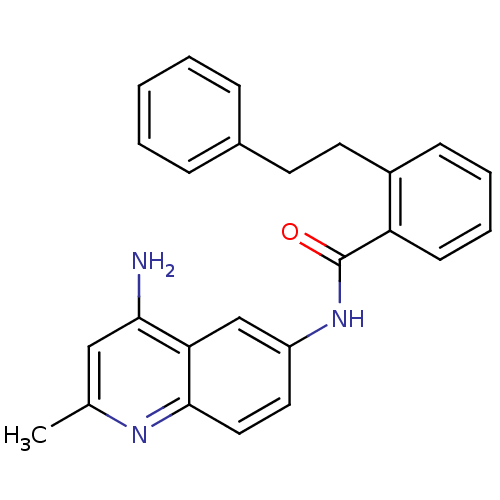
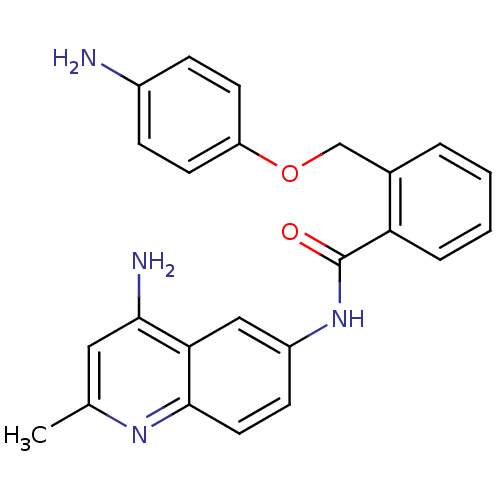
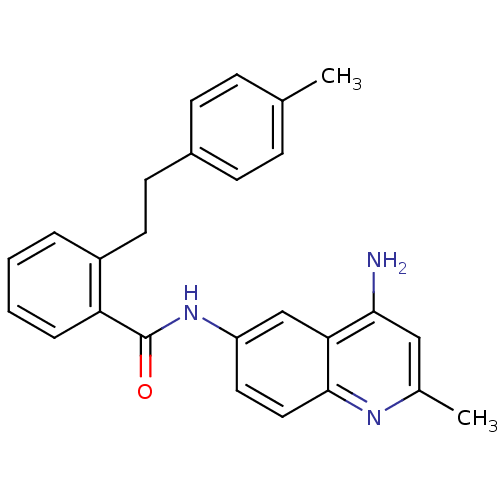
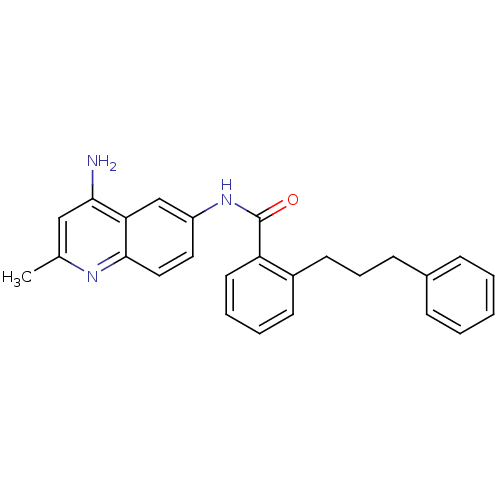
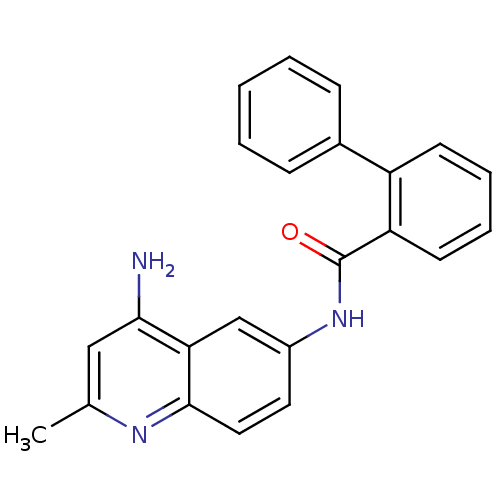
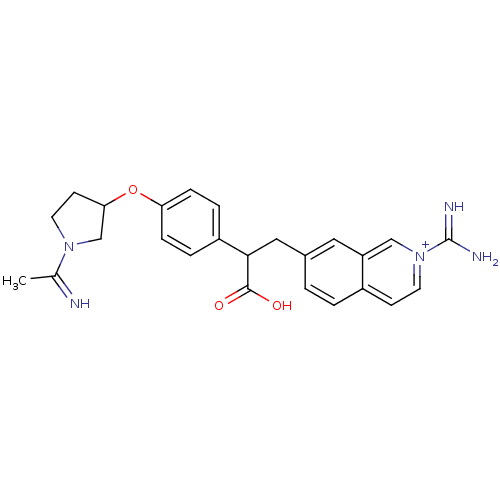
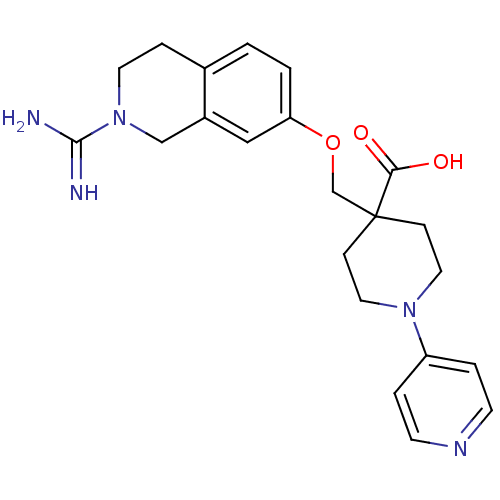
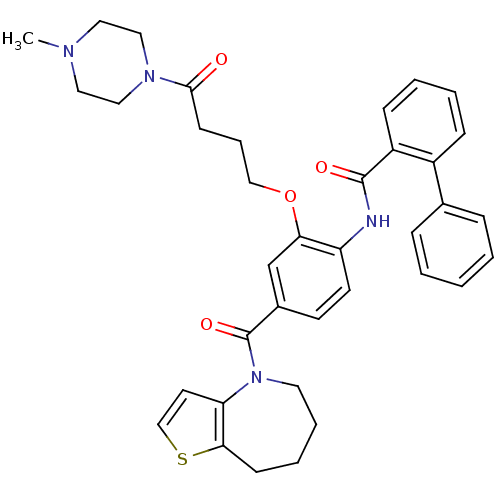
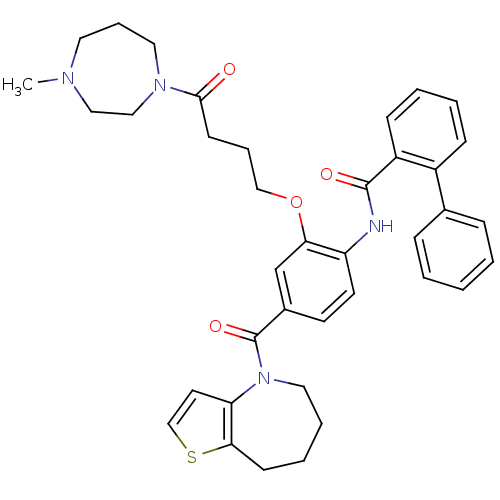
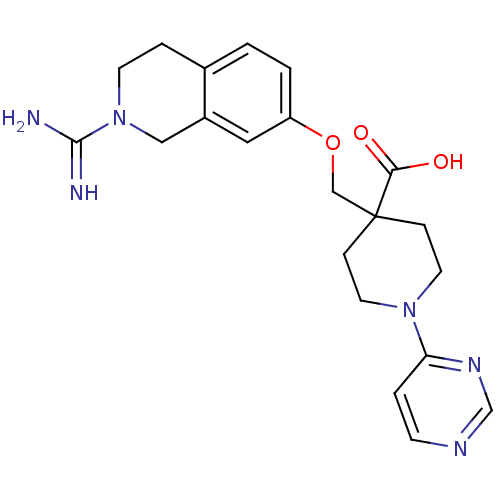
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
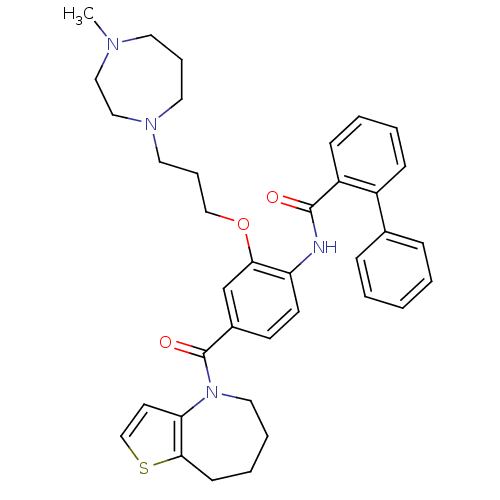
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
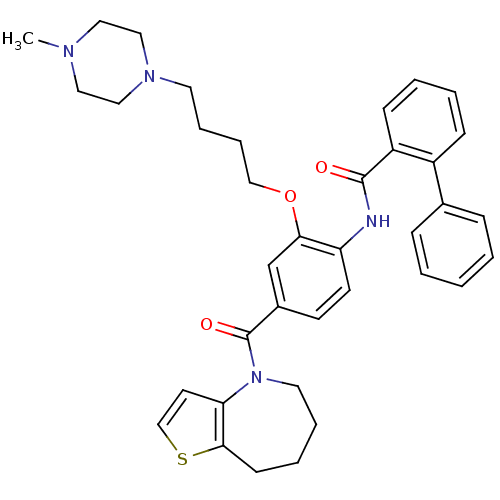
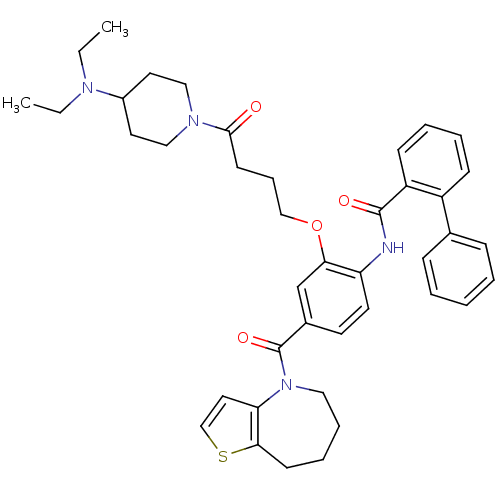
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
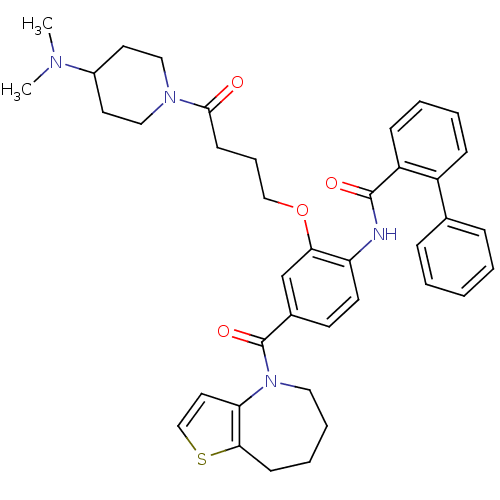
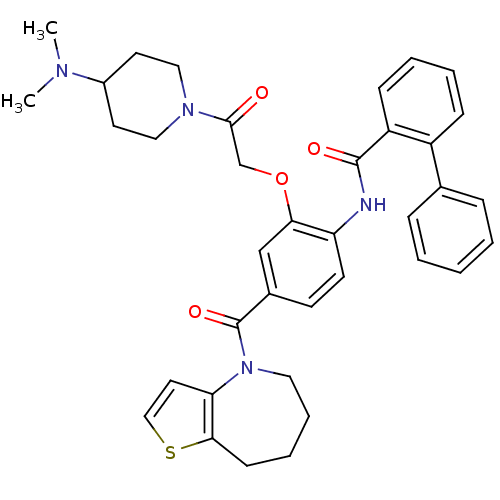
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 2.20nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 2.60nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 6.5nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 7nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 8.20nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
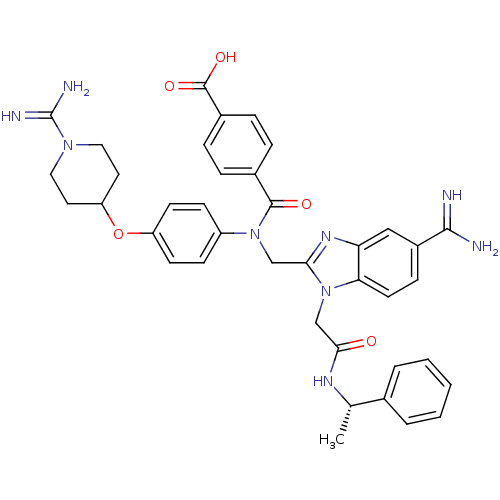
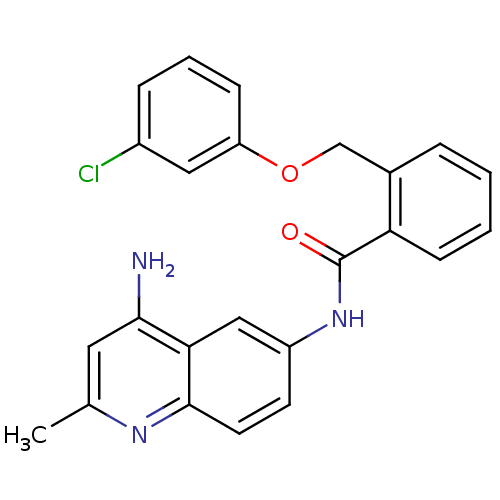
TargetCoagulation factor X(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 19nMAssay Description:Inhibitory concentration against human Coagulation factor XMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 37nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
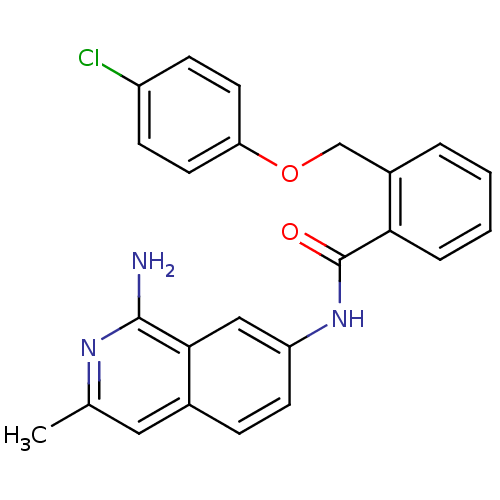
TargetCoagulation factor X(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 41nMAssay Description:Inhibitory concentration against human Coagulation factor XMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 47nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 51nMAssay Description:Compound was evaluated for its ability to displace [3H]-nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 80nMAssay Description:Compound was evaluated for its ability to displace [3H]-nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 82nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 86nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 89nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 103nMAssay Description:Inhibition of [3H]diprenorphine (0.33 nM) binding from human Opioid receptor mu 1 expressed in CHO-K1 cells.More data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 121nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 369nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetSerine protease 1/Trypsin-2(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 620nMAssay Description:Inhibitory concentration against human TrypsinMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.06E+3nMAssay Description:Inhibition of [3H]naltrindole (0.55 nM) binding from human Opioid receptor kappa 1More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 8.65E+3nMAssay Description:Inhibition of [3H]naltrindole (0.55 nM) binding from human Opioid receptor delta 1 expressed in CHO-K1 cells.More data for this Ligand-Target Pair
TargetSerine protease 1/Trypsin-2(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.36E+4nMAssay Description:Inhibitory concentration against human TrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 2.30E+4nMAssay Description:Mean inhibitory concentration against plasmin; n=3More data for this Ligand-Target Pair
Affinity DataKi: 2.30E+4nMAssay Description:Inhibitory concentration against human PlasminMore data for this Ligand-Target Pair
Affinity DataKi: 7.82E+4nMAssay Description:Inhibitory concentration against human PlasminMore data for this Ligand-Target Pair
Affinity DataKi: 7.82E+4nMAssay Description:Mean inhibitory concentration against plasmin; n=3More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibitory concentration against human Coagulation factor II (thrombin)More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibitory concentration against human Coagulation factor II (thrombin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.680nMAssay Description:In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50More data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Rattus norvegicus (Rat))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibitory activity against arginine vasopressin V2 receptor using [3H]-AVP as radioligand in rat adrenal medullaMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Rattus norvegicus (Rat))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: <1nMAssay Description:Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medullaMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]-AVP as radioligand in CHO cellsMore data for this Ligand-Target Pair
TargetVasopressin V1a/V1b receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: <1nMAssay Description:Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in human plateletMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in rat liverMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]-AVP as radioligand in CHO cellsMore data for this Ligand-Target Pair
TargetVasopressin V1a/V1b receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in human plateletMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Rattus norvegicus (Rat))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibitory activity against arginine vasopressin V2 receptor using [3H]-AVP as radioligand in rat adrenal medullaMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Rattus norvegicus (Rat))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibitory activity against arginine vasopressin V2 receptor using [3H]-AVP as radioligand in rat adrenal medullaMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in rat liverMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibitory concentration against human Coagulation factor XMore data for this Ligand-Target Pair
TargetVasopressin V1a/V1b receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in human plateletMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Rattus norvegicus (Rat))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibitory activity against arginine vasopressin V2 receptor using [3H]-AVP as radioligand in rat adrenal medullaMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in rat liverMore data for this Ligand-Target Pair
TargetVasopressin V1a/V1b receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in human plateletMore data for this Ligand-Target Pair

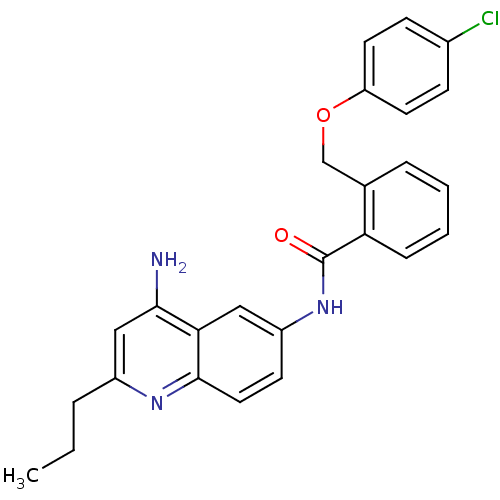
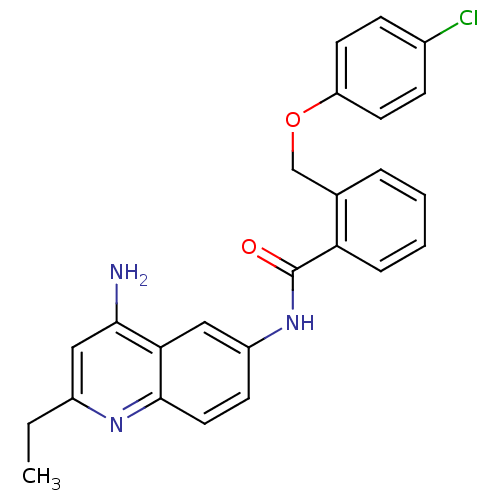
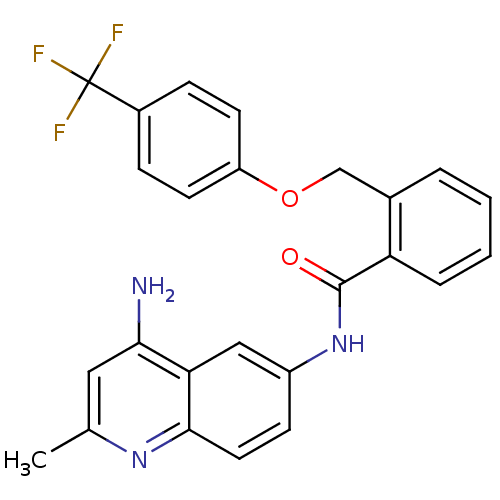
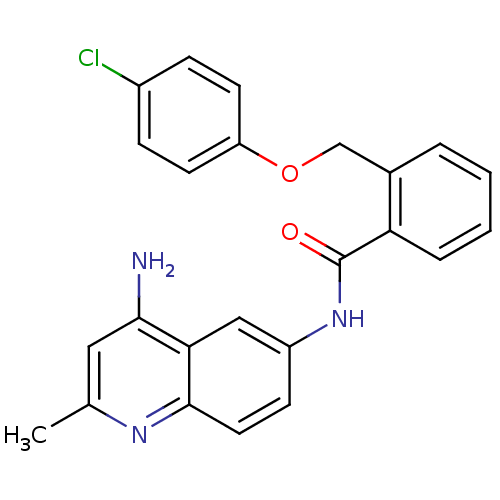

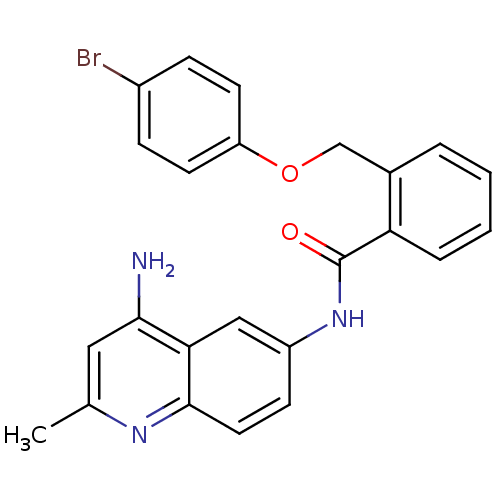
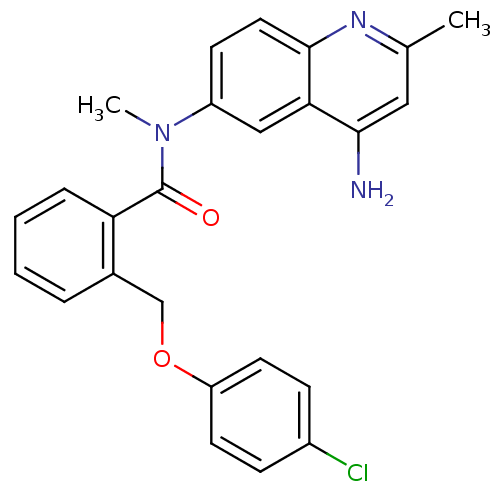

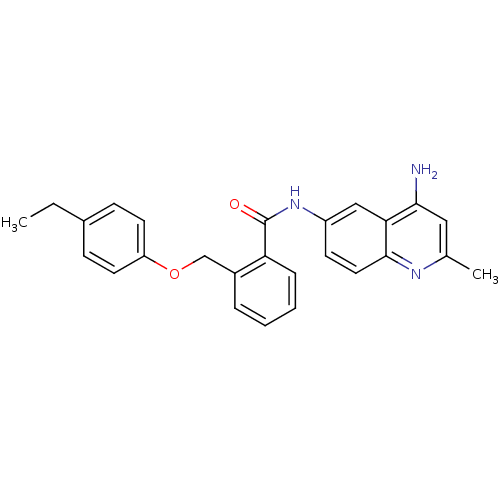

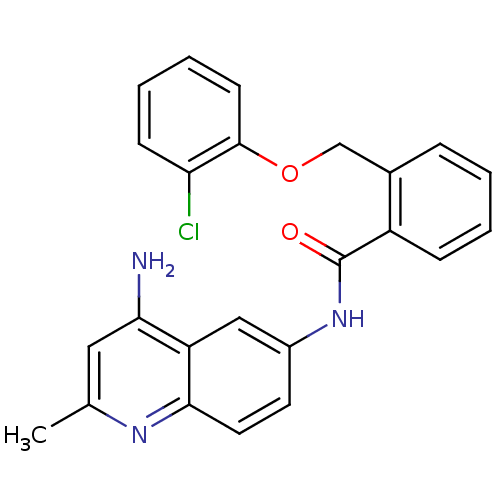

 3D Structure (crystal)
3D Structure (crystal)