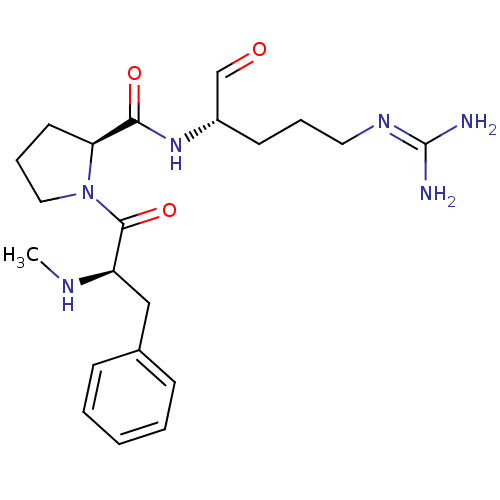
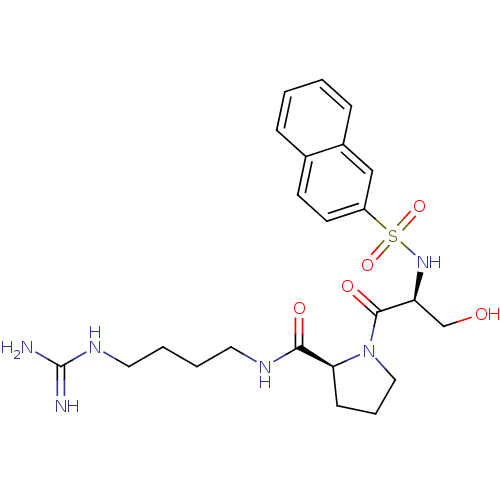
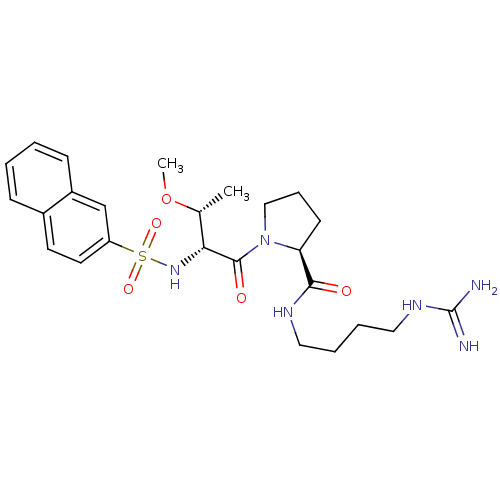
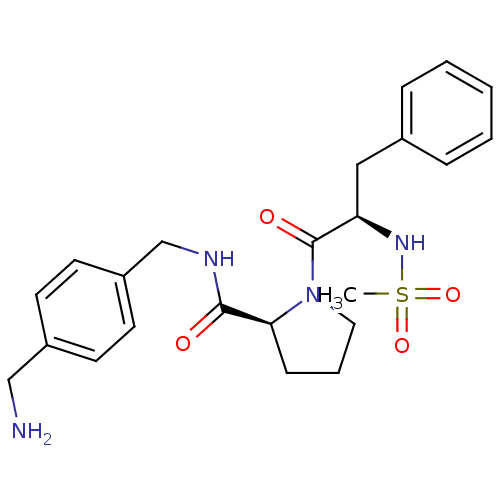
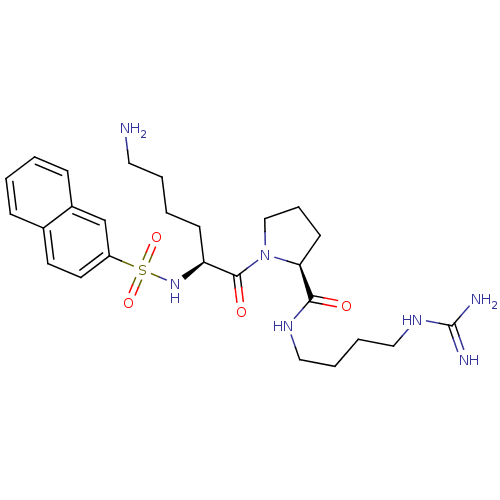
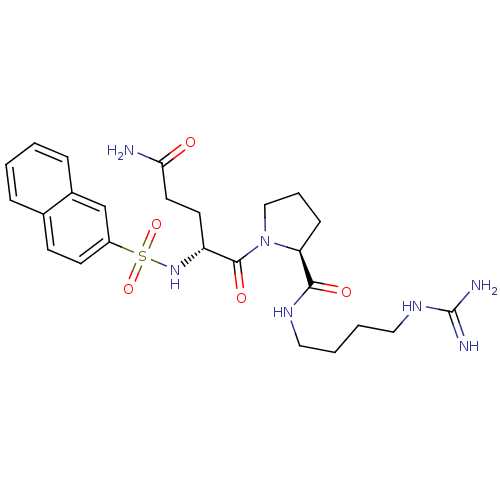
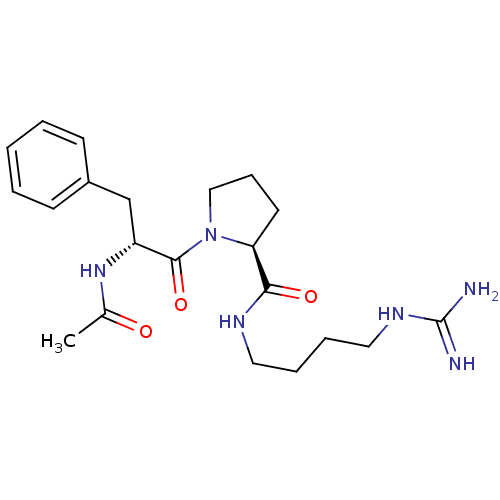
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.460nMAssay Description:Competitive kinetic for thrombin inhibition Ki was determinedMore data for this Ligand-Target Pair
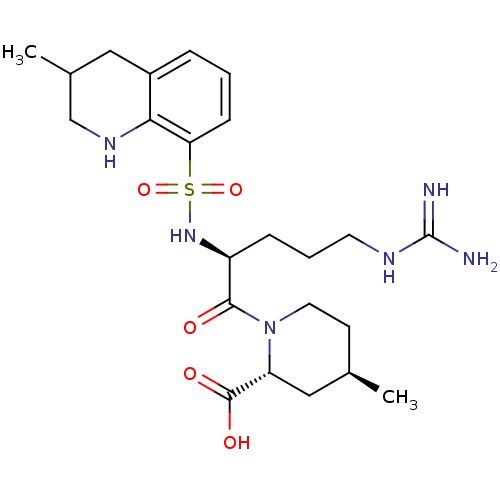
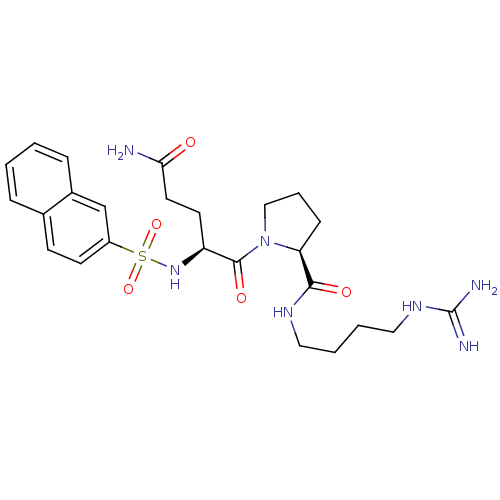
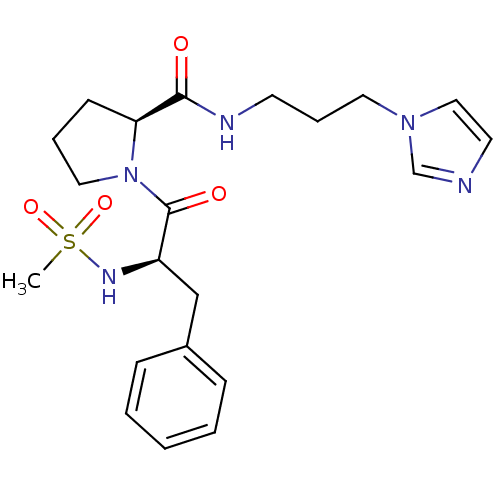
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 8.20nMAssay Description:Competitive kinetic for human alpha thrombin inhibition Ki was determinedMore data for this Ligand-Target Pair
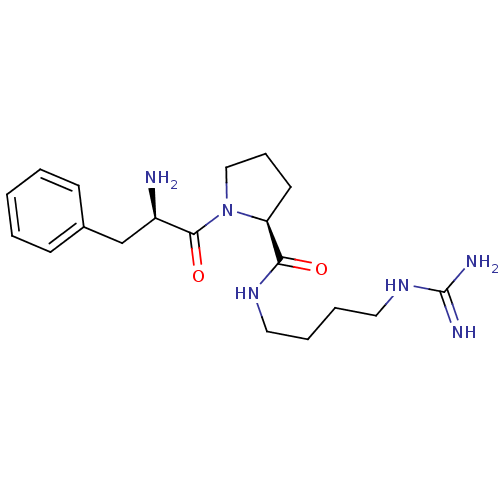
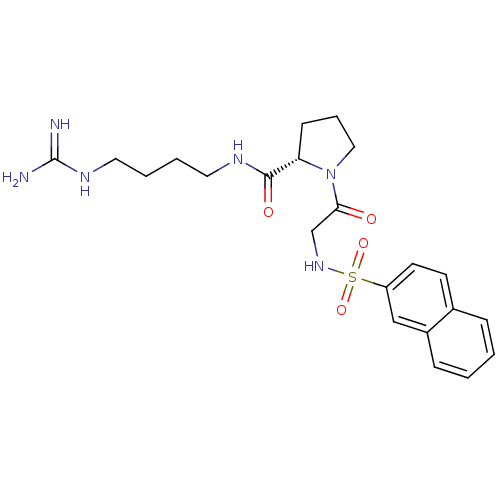
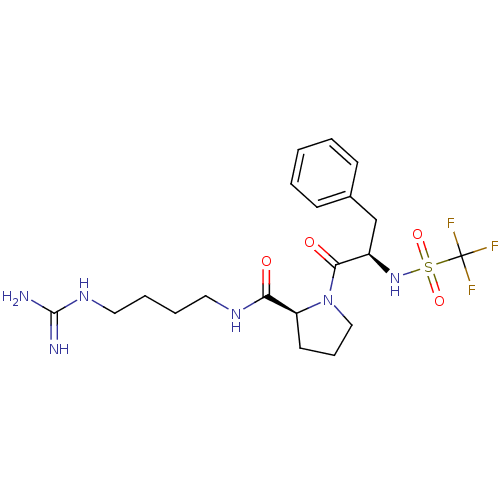
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:In vitro inhibitory activity against hydrolysis of thrombin was determinedMore data for this Ligand-Target Pair
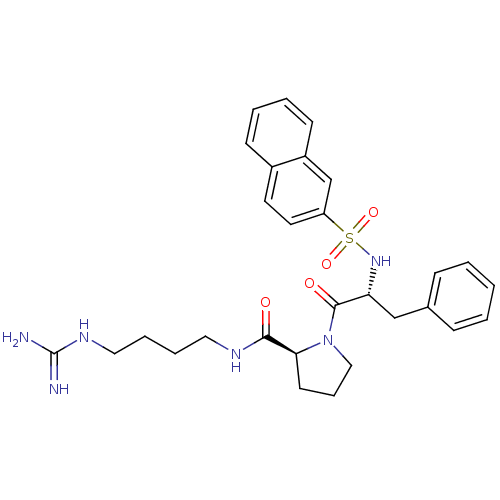
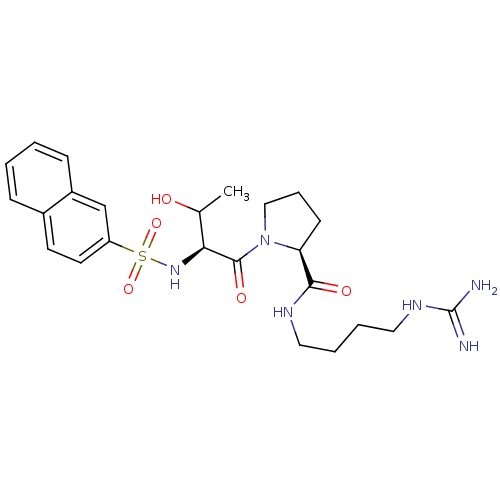
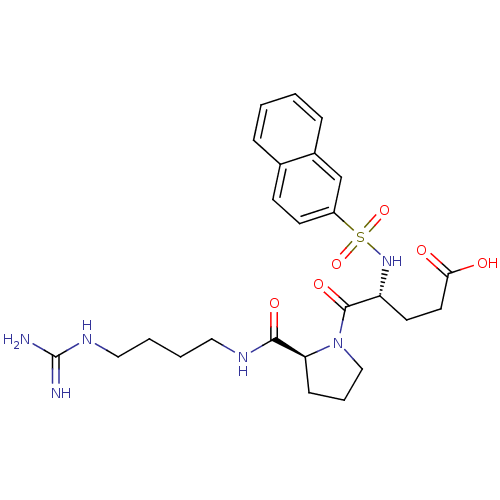
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 46nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 540nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 670nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.90E+3nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.90E+3nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.50E+3nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.40E+3nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.90E+3nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.20E+3nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 8.90E+3nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.20E+4nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.66E+4nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.20E+4nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.08E+5nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.10E+5nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.34E+5nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: >3.33E+5nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair

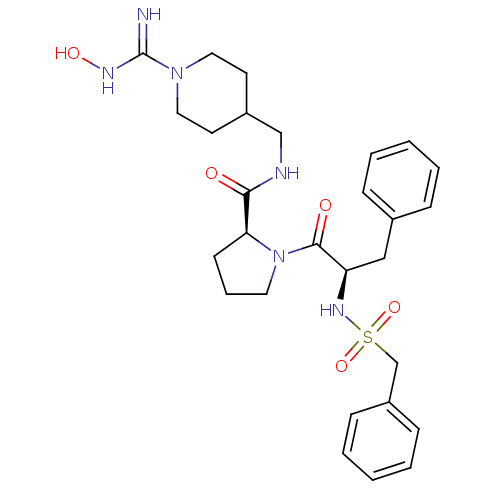
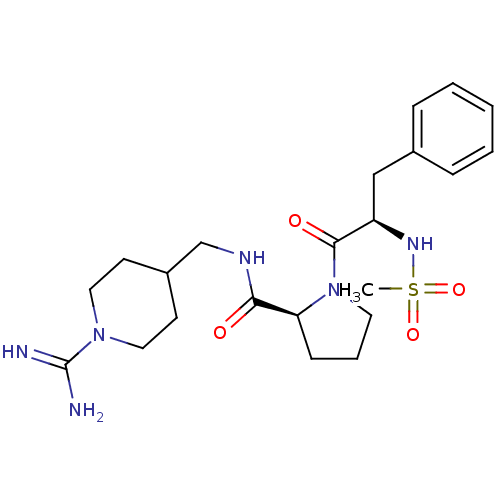
 3D Structure (crystal)
3D Structure (crystal)