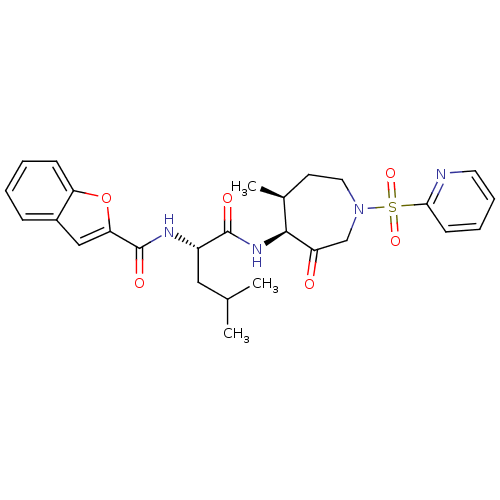
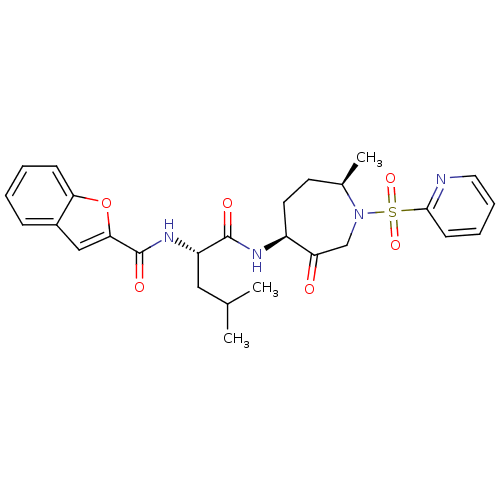
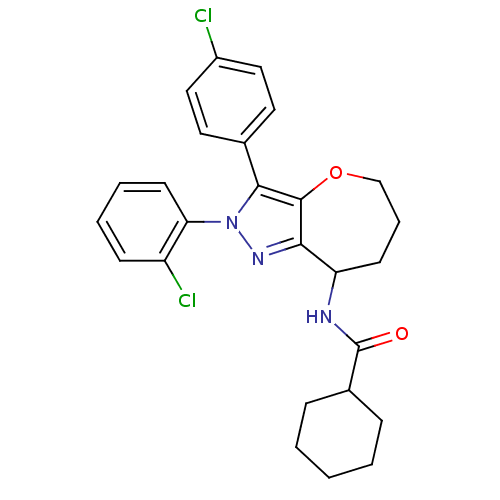
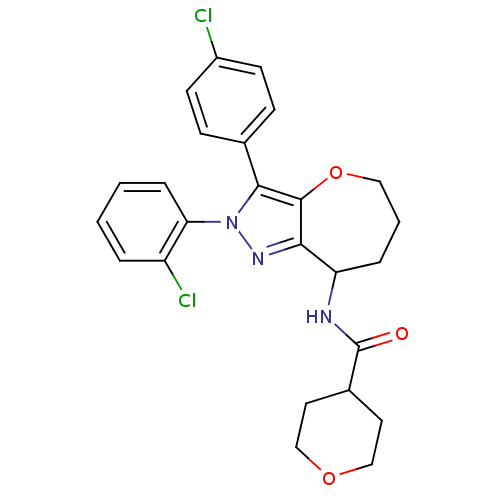
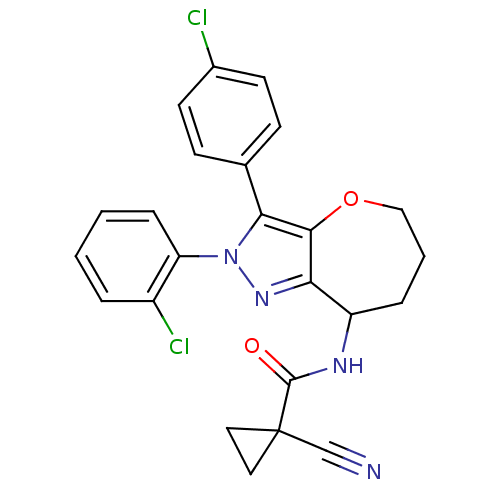
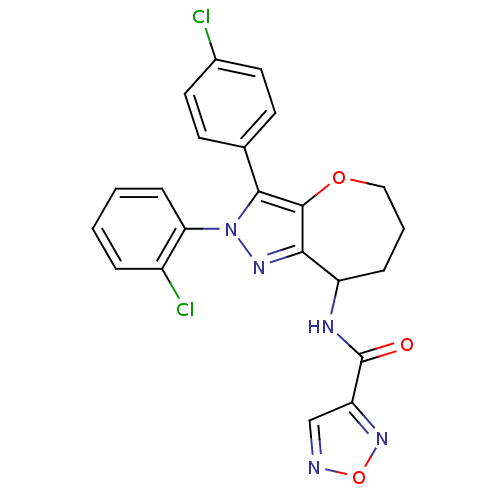
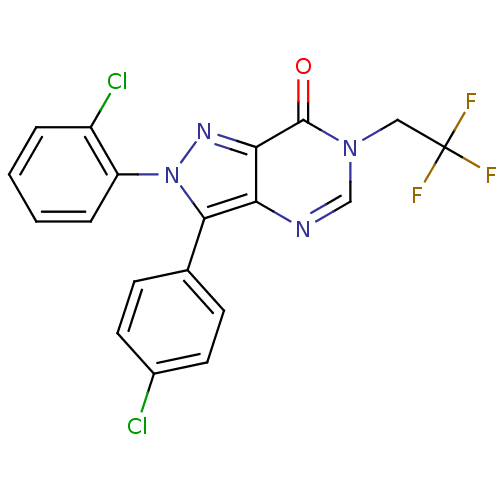
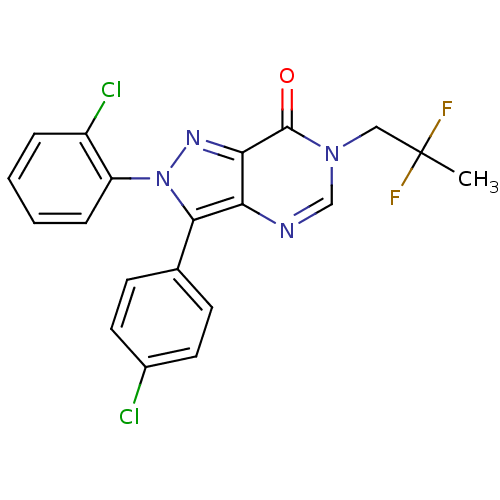
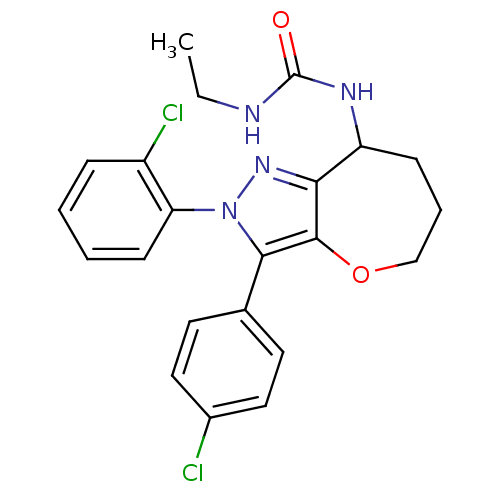
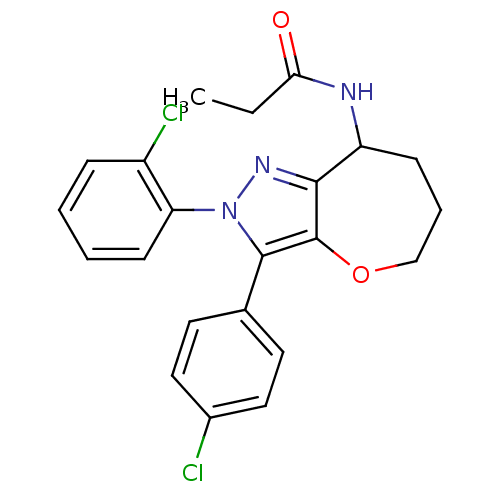
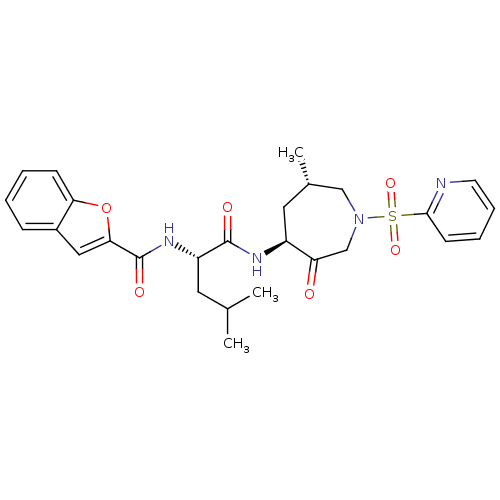
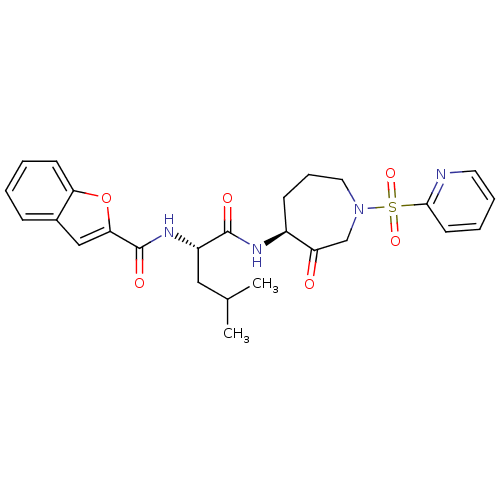
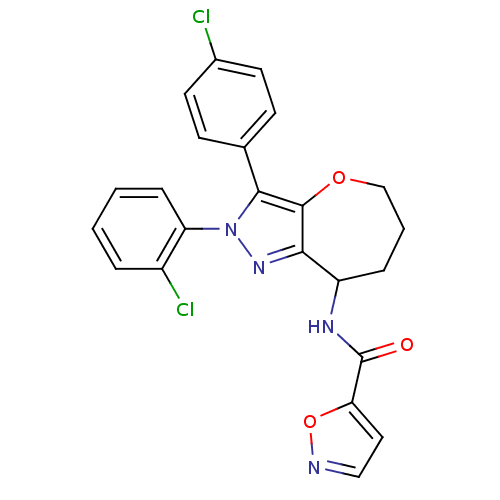
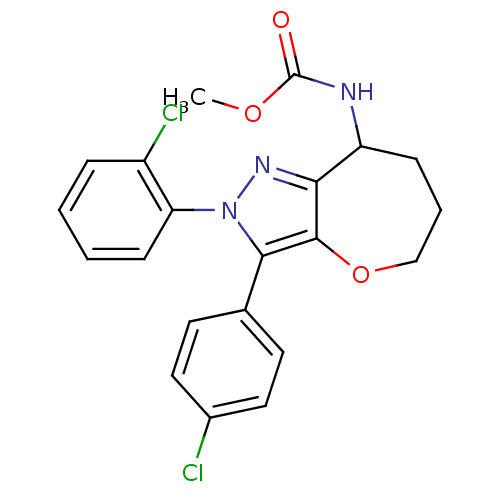
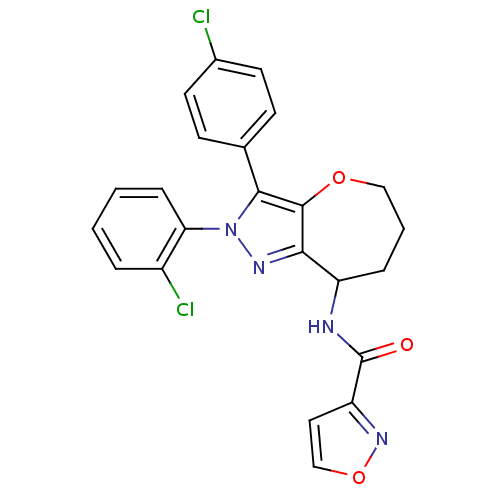
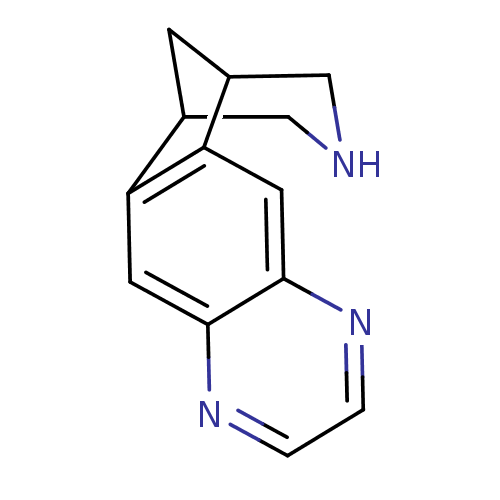
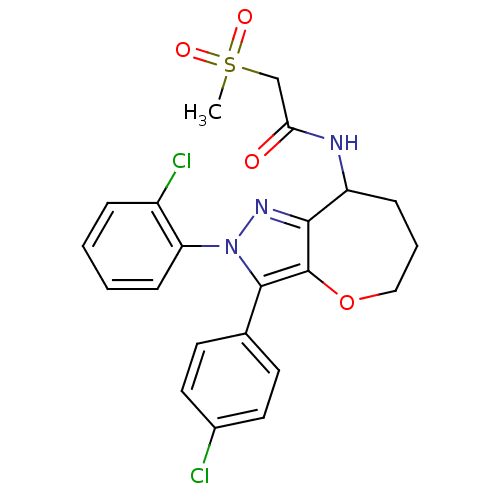
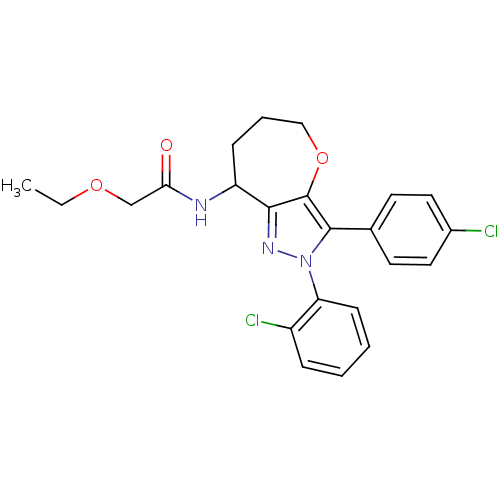
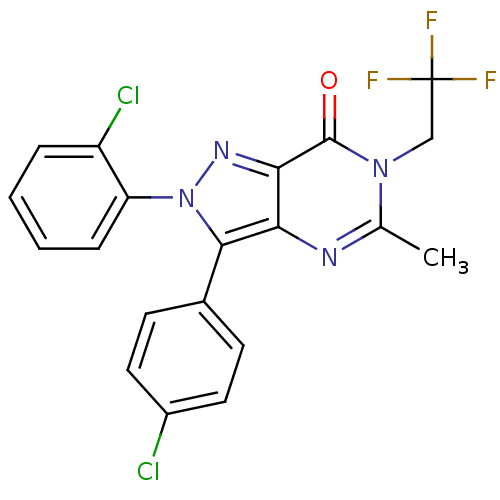
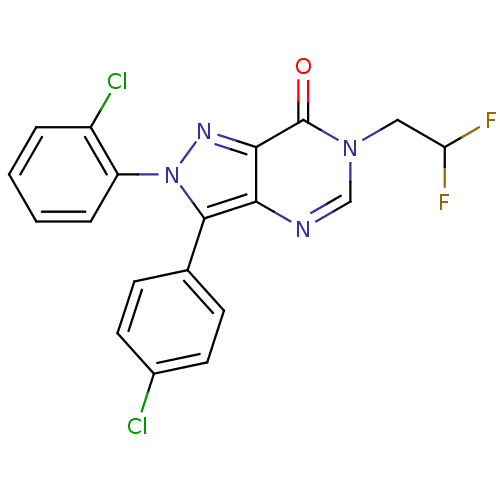
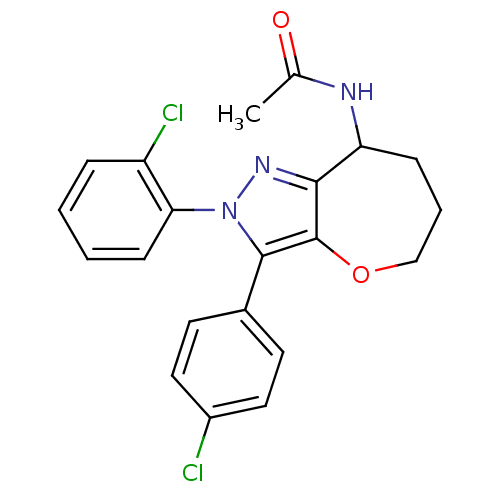
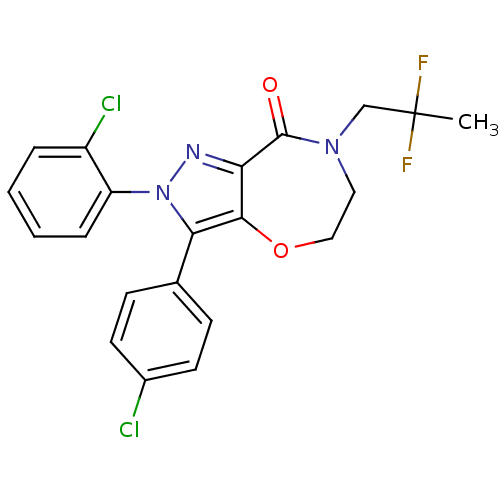
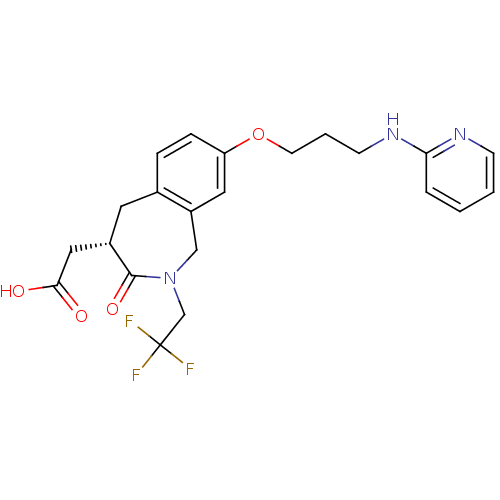
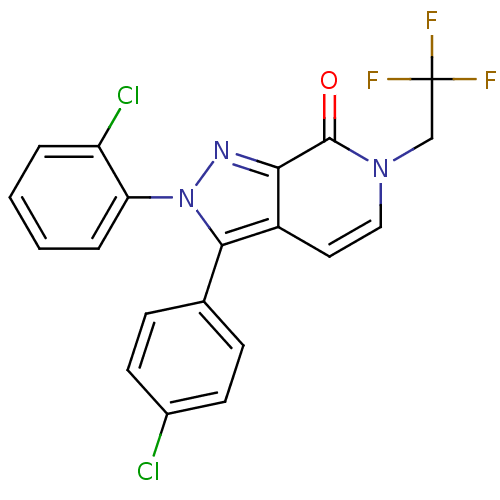
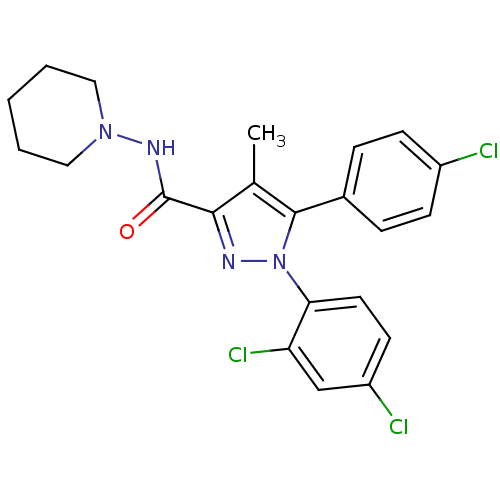
Affinity DataKi: 0.00480nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 0.00990nM ΔG°: -62.2kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.0300nMAssay Description:Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1...More data for this Ligand-Target Pair
Affinity DataKi: 0.0400nMAssay Description:Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1...More data for this Ligand-Target Pair
Affinity DataKi: 0.0400nM ΔG°: -58.8kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.0410nM ΔG°: -58.7kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.0500nMAssay Description:Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1...More data for this Ligand-Target Pair
Affinity DataKi: 0.0630nMAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.0680nM ΔG°: -57.4kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.0700nMAssay Description:Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1...More data for this Ligand-Target Pair
Affinity DataKi: 0.0800nMAssay Description:Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1...More data for this Ligand-Target Pair
Affinity DataKi: 0.0800nMAssay Description:Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1...More data for this Ligand-Target Pair
Affinity DataKi: 0.0900nMAssay Description:Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1...More data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1...More data for this Ligand-Target Pair
Affinity DataKi: 0.120nMAssay Description:Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1...More data for this Ligand-Target Pair
Affinity DataKi: 0.120nMAssay Description:Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.130nMAssay Description:Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.140nM ΔG°: -55.7kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.160nM ΔG°: -55.3kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.230nMAssay Description:Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1...More data for this Ligand-Target Pair
Affinity DataKi: 0.25nMAssay Description:Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.260nMAssay Description:Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1...More data for this Ligand-Target Pair
Affinity DataKi: 0.260nMAssay Description:Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta countingMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4/beta-2(Homo sapiens (Human))
Pfizer
Curated by ChEMBL
Pfizer
Curated by ChEMBL
Affinity DataKi: 0.295nMAssay Description:Displacement of [3H]epibatidine from alpha4beta2 nicotinic receptor expressed in human HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.410nMAssay Description:Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.490nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 0.540nMAssay Description:Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.550nMAssay Description:Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.560nMAssay Description:Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.590nMAssay Description:Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.630nM ΔG°: -52.0kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.720nMAssay Description:Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1...More data for this Ligand-Target Pair
Affinity DataKi: 0.75nMAssay Description:Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.820nMAssay Description:Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1...More data for this Ligand-Target Pair
Affinity DataKi: 0.820nMAssay Description:Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1...More data for this Ligand-Target Pair
Affinity DataKi: 0.870nMAssay Description:Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1...More data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Binding affinity for Vitronectin receptor (alpha V beta 3)More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assayMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assayMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)