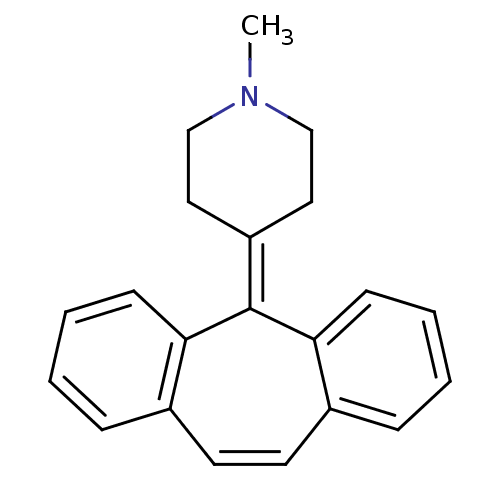
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
National Institute Of Biological Sciences
Curated by ChEMBL
National Institute Of Biological Sciences
Curated by ChEMBL
Affinity DataKi: 0.460nMAssay Description:Antagonist activity at 5HT2A receptorMore data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
National Institute Of Biological Sciences
Curated by ChEMBL
National Institute Of Biological Sciences
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Antagonist activity at histamine H1 receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
National Institute Of Biological Sciences
Curated by ChEMBL
National Institute Of Biological Sciences
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Antagonist activity at 5HT2A receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2B(Homo sapiens (Human))
National Institute Of Biological Sciences
Curated by ChEMBL
National Institute Of Biological Sciences
Curated by ChEMBL
Affinity DataKi: 56nMAssay Description:Displacement of [3H]LSD from 5HT2B receptor after 1.5 hrs by scintillation counterMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
National Institute Of Biological Sciences
Curated by ChEMBL
National Institute Of Biological Sciences
Curated by ChEMBL
Affinity DataKi: 417nMAssay Description:Displacement of [3H]LSD from 5HT2C receptor after 1.5 hrs by scintillation counterMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Homo sapiens (Human))
National Institute Of Biological Sciences
Curated by ChEMBL
National Institute Of Biological Sciences
Curated by ChEMBL
Affinity DataKi: 1.18E+3nMAssay Description:Displacement of [3H]8-OH-DPAT from 5HT1A receptor after 1.5 hrs by scintillation counterMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
National Institute Of Biological Sciences
Curated by ChEMBL
National Institute Of Biological Sciences
Curated by ChEMBL
Affinity DataKi: 1.96E+3nMAssay Description:Displacement of [3H]Ketanserin from 5HT2A receptor after 1.5 hrs by scintillation counterMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 5A(Homo sapiens (Human))
National Institute Of Biological Sciences
Curated by ChEMBL
National Institute Of Biological Sciences
Curated by ChEMBL
Affinity DataKi: 3.30E+3nMAssay Description:Displacement of [3H]LSD from 5HT5A receptor after 1.5 hrs by scintillation counterMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 6(Homo sapiens (Human))
National Institute Of Biological Sciences
Curated by ChEMBL
National Institute Of Biological Sciences
Curated by ChEMBL
Affinity DataKi: 6.21E+3nMAssay Description:Displacement of [3H]LSD from 5HT6 receptor after 1.5 hrs by scintillation counterMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 7(Homo sapiens (Human))
National Institute Of Biological Sciences
Curated by ChEMBL
National Institute Of Biological Sciences
Curated by ChEMBL
Affinity DataKi: 7.07E+3nMAssay Description:Displacement of [3H]LSD from 5HT7 receptor after 1.5 hrs by scintillation counterMore data for this Ligand-Target Pair
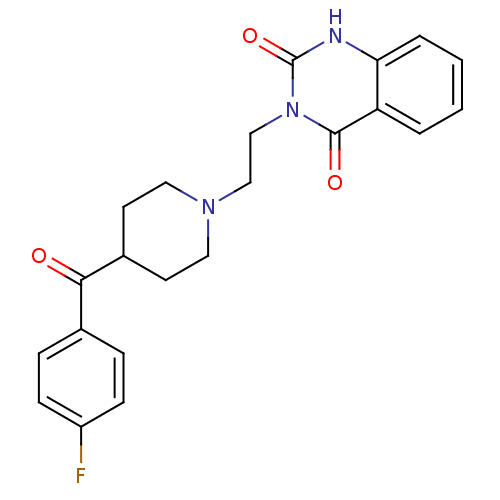
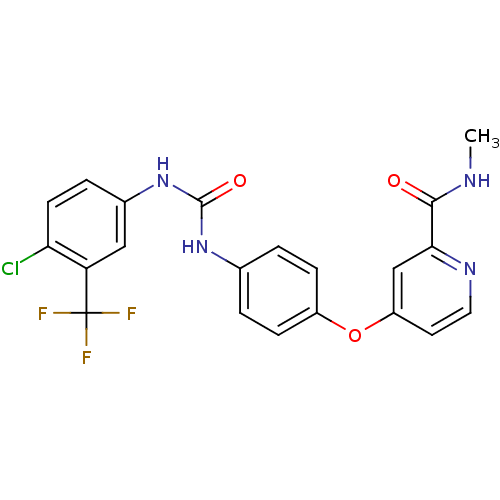
TargetVascular endothelial growth factor receptor 3(Homo sapiens (Human))
National Institute Of Biological Sciences
Curated by ChEMBL
National Institute Of Biological Sciences
Curated by ChEMBL
TargetMast/stem cell growth factor receptor Kit(Homo sapiens (Human))
National Institute Of Biological Sciences
Curated by ChEMBL
National Institute Of Biological Sciences
Curated by ChEMBL
TargetVascular endothelial growth factor receptor 1(Homo sapiens (Human))
National Institute Of Biological Sciences
Curated by ChEMBL
National Institute Of Biological Sciences
Curated by ChEMBL
TargetPlatelet-derived growth factor receptor beta(Homo sapiens (Human))
National Institute Of Biological Sciences
Curated by ChEMBL
National Institute Of Biological Sciences
Curated by ChEMBL
Affinity DataIC50: 37nMAssay Description:Inhibition of PDGFRbetaMore data for this Ligand-Target Pair
TargetRAF proto-oncogene serine/threonine-protein kinase(Homo sapiens (Human))
National Institute Of Biological Sciences
Curated by ChEMBL
National Institute Of Biological Sciences
Curated by ChEMBL
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
National Institute Of Biological Sciences
Curated by ChEMBL
National Institute Of Biological Sciences
Curated by ChEMBL

 3D Structure (crystal)
3D Structure (crystal)