Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB (change energy unit to kcal/mol)
 Found 10 hits in this display
Found 10 hits in this display
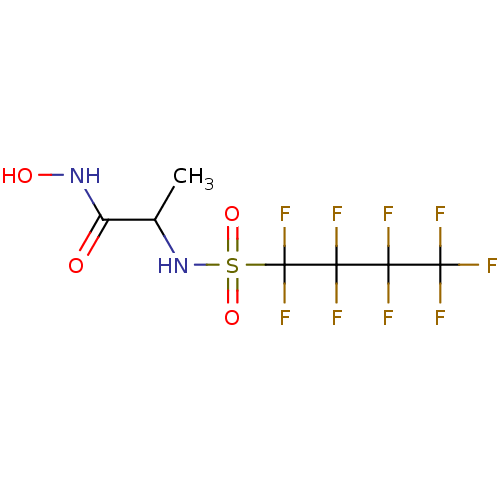 BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)
BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)
 BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)
BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)
 BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)
BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)
 BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)
BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)
 BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)
BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)
 BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)
BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)
 BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)
BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)
 BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)
BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)
 BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)
BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)
 BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)
BDBM11332(Hydroxamate 13 | N-hydroxy-2-[(1,1,2,2,3,3,4,4,4-n...)