Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB (change energy unit to kcal/mol)
 Found 20 hits in this display
Found 20 hits in this display
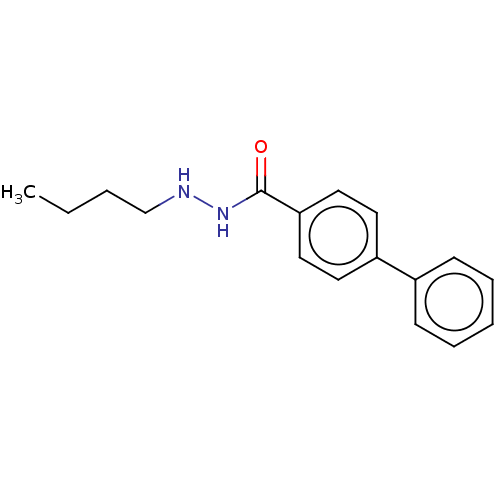 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)IC50: 90nMAssay Description:These SAR data indicate that a tripartite structure of this scaffold with a central —C(O)—NH—NH— unit flanked by a phenyl group and a short aliphatic...More data for this Ligand-Target Pair
 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)IC50: 90nMAssay Description:These SAR data indicate that a tripartite structure of this scaffold with a central C(O) NH NH unit flanked by a phenyl group and a short aliphatic c...More data for this Ligand-Target Pair
 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)IC50: 800nMAssay Description:These SAR data indicate that a tripartite structure of this scaffold with a central —C(O)—NH—NH— unit flanked by a phenyl group and a short aliphatic...More data for this Ligand-Target Pair
 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)IC50: 800nMAssay Description:Inhibition of C-terminal 6xHis-tagged full-length recombinant human HDAC2 (1 to 488 residues) expressed in Sf9 insect cells by Luminescence Glo assayMore data for this Ligand-Target Pair
 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
 BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)
BDBM468609(US10807944, Compound RLS3-43 | US10870618, Compoun...)IC50: 2.43E+3nMAssay Description:These SAR data indicate that a tripartite structure of this scaffold with a central —C(O)—NH—NH— unit flanked by a phenyl group and a short aliphatic...More data for this Ligand-Target Pair