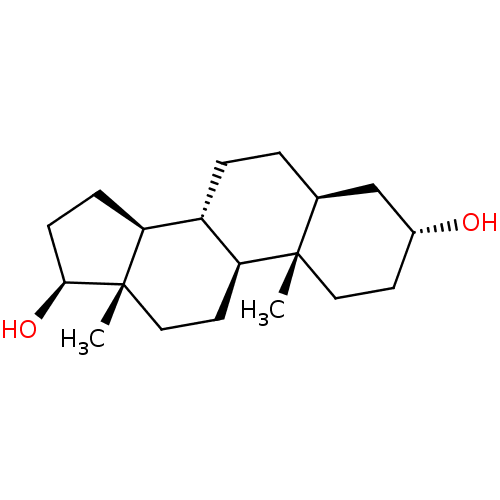
 hombreol 3alpha,17beta-dihydroxy-5alpha-androstane 5alpha-androstane-3alpha,17beta-diol (3alpha,5alpha,17beta)-androstane-3,17-diol CHEMBL335062 BDBM50093445
hombreol 3alpha,17beta-dihydroxy-5alpha-androstane 5alpha-androstane-3alpha,17beta-diol (3alpha,5alpha,17beta)-androstane-3,17-diol CHEMBL335062 BDBM50093445 CHEMBL260660 BDBM50423533 Dihydroequilenin-17Beta
CHEMBL260660 BDBM50423533 Dihydroequilenin-17Beta 3beta,17beta-Dihydroxyandrost-5-ene CHEMBL440283 Androstenediol (3beta,17beta)-androst-5-ene-3,17-diol 3beta,17beta-Dihydroxy-5-androstene hermaphrodiol Androst-5-ene-3beta,17beta-diol androst-5-enediol BDBM50223237
3beta,17beta-Dihydroxyandrost-5-ene CHEMBL440283 Androstenediol (3beta,17beta)-androst-5-ene-3,17-diol 3beta,17beta-Dihydroxy-5-androstene hermaphrodiol Androst-5-ene-3beta,17beta-diol androst-5-enediol BDBM50223237 17beta-hydroxy-4-estren-3-one 17beta-hydroxyestr-4-en-3-one CHEMBL757 Nandrolone BDBM50080092 (17beta)-17-hydroxyestr-4-en-3-one 19-Nortestosterone 19-Norandrostenolone 4-estren-17beta-ol-3-one 17beta-hydroxy-19-nor-4-androsten-3-one
17beta-hydroxy-4-estren-3-one 17beta-hydroxyestr-4-en-3-one CHEMBL757 Nandrolone BDBM50080092 (17beta)-17-hydroxyestr-4-en-3-one 19-Nortestosterone 19-Norandrostenolone 4-estren-17beta-ol-3-one 17beta-hydroxy-19-nor-4-androsten-3-one 17Beta-HSD Inhibitor, 3 BDBM85468
17Beta-HSD Inhibitor, 3 BDBM85468 BDBM50423526 CHEMBL121458 17Beta-Dihydroequillin 7-Dehydro-Estradiol
BDBM50423526 CHEMBL121458 17Beta-Dihydroequillin 7-Dehydro-Estradiol 3ALPHA,17BETA-DIHYDROXY-5BETA-ANDROSTAN-4-ONE BDBM50441293
3ALPHA,17BETA-DIHYDROXY-5BETA-ANDROSTAN-4-ONE BDBM50441293 BDBM91715 17beta-Hydroxy-5alpha-androstan-3,6-dione, 4
BDBM91715 17beta-Hydroxy-5alpha-androstan-3,6-dione, 4 19-Norethisterone Primolut-N BDBM50148732 17alpha-ethinylestra-4-en-17beta-ol-3-one 19-Nor-17alpha-ethynyl-17beta-hydroxy-4-androsten-3-one Norethisteron NORETHINDRONE norethisterone 17alpha-ethynyl-19-nor-4-androsten-17beta-ol-3-one 19-nor-17alpha-ethynyltestosterone 4-estren-17alpha-ethynyl-17beta-ol-3-one 17alpha-ethinyl-19-nortestosterone 17-ethynyl-17beta-hydroxyestr-4-en-3-one CHEMBL1162 17beta-hydroxy-19-norpregn-4-en-20-yn-3-one Micronor
19-Norethisterone Primolut-N BDBM50148732 17alpha-ethinylestra-4-en-17beta-ol-3-one 19-Nor-17alpha-ethynyl-17beta-hydroxy-4-androsten-3-one Norethisteron NORETHINDRONE norethisterone 17alpha-ethynyl-19-nor-4-androsten-17beta-ol-3-one 19-nor-17alpha-ethynyltestosterone 4-estren-17alpha-ethynyl-17beta-ol-3-one 17alpha-ethinyl-19-nortestosterone 17-ethynyl-17beta-hydroxyestr-4-en-3-one CHEMBL1162 17beta-hydroxy-19-norpregn-4-en-20-yn-3-one Micronor 11alpha-17beta-Dihydroxyandrost-1,4-dien-3-one, 11 BDBM91722
11alpha-17beta-Dihydroxyandrost-1,4-dien-3-one, 11 BDBM91722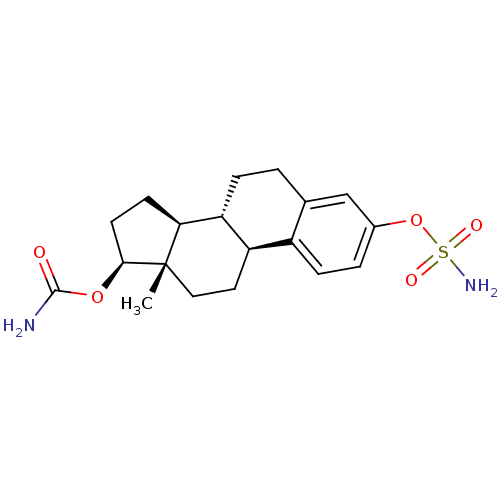 CHEMBL229993 17beta-carbamoyloxy-3-sulfamoyloxyestra-1,3,5(10)-triene BDBM50219531
CHEMBL229993 17beta-carbamoyloxy-3-sulfamoyloxyestra-1,3,5(10)-triene BDBM50219531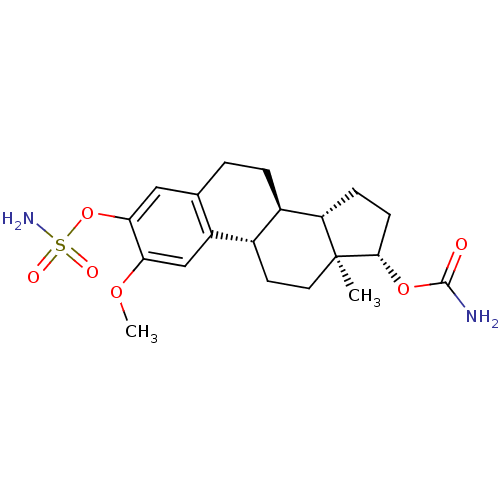 17beta-Carbamoyloxy-2-methoxy-3-sulfamoyloxyestra-1,3,5(10)-triene BDBM50219532 CHEMBL389320
17beta-Carbamoyloxy-2-methoxy-3-sulfamoyloxyestra-1,3,5(10)-triene BDBM50219532 CHEMBL389320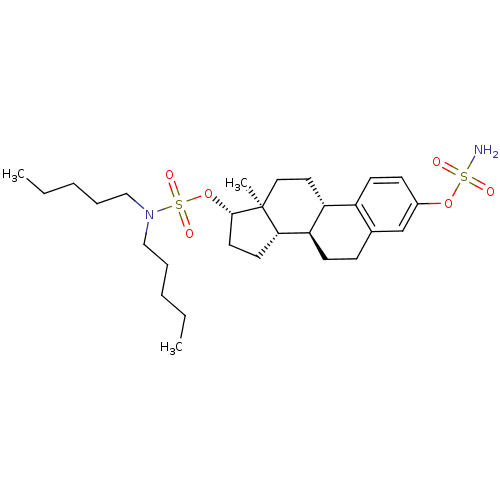 CHEMBL385226 3-O-sulfamoylestradiol 17beta-O-(N,N-dipentyl)sulfamate BDBM50200942
CHEMBL385226 3-O-sulfamoylestradiol 17beta-O-(N,N-dipentyl)sulfamate BDBM50200942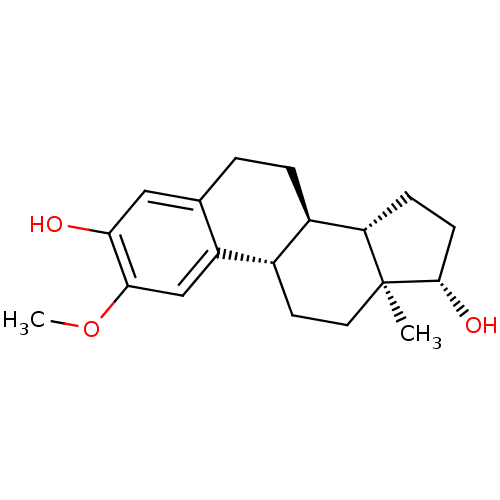 2-methoxy-17beta-estradiol CHEMBL299613 BDBM50060957 Panzem 2-Hydroxyestradol 2-methyl ether
2-methoxy-17beta-estradiol CHEMBL299613 BDBM50060957 Panzem 2-Hydroxyestradol 2-methyl ether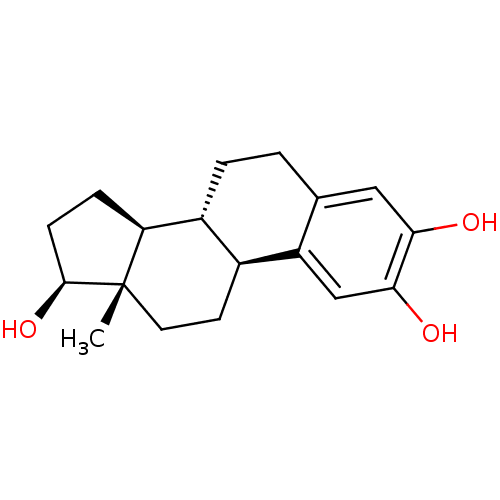 2-OH-estradiol 2-hydroxy-17beta-estradiol cid_247304 (17beta)-estra-1,3,5(10)-triene-2,3,17-triol US10864248, Compound 2 2-OH-E2 BDBM50262140 CHEMBL467987 estra-1,3,5(10)-triene-2,3,17beta-triol
2-OH-estradiol 2-hydroxy-17beta-estradiol cid_247304 (17beta)-estra-1,3,5(10)-triene-2,3,17-triol US10864248, Compound 2 2-OH-E2 BDBM50262140 CHEMBL467987 estra-1,3,5(10)-triene-2,3,17beta-triol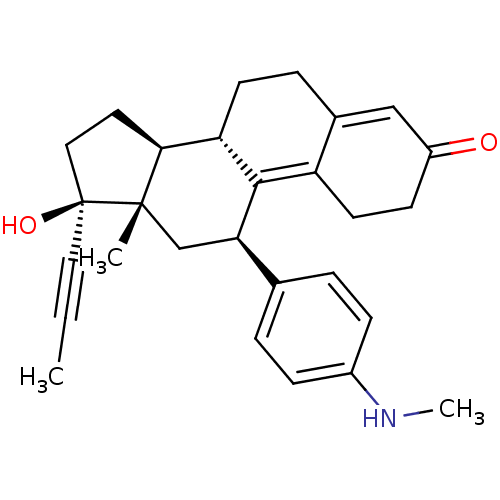 BDBM50292750 17beta-Hydroxy-11beta-[4-(methylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL1608 17beta-hydroxy-17alpha-propynyl-11beta-(4-N-methylaminophenyl)-estra-4,9-dien-3-one
BDBM50292750 17beta-Hydroxy-11beta-[4-(methylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL1608 17beta-hydroxy-17alpha-propynyl-11beta-(4-N-methylaminophenyl)-estra-4,9-dien-3-one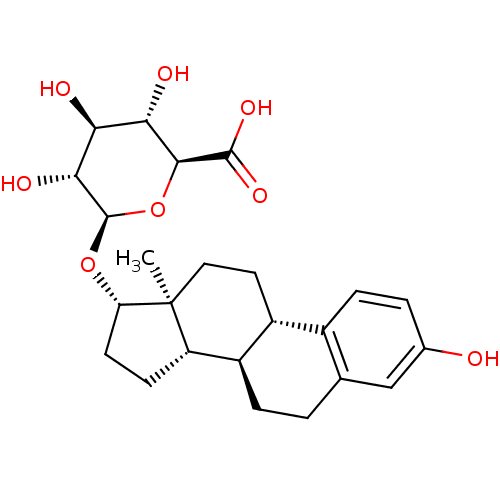 17beta-Estradiol-17-(beta-D-glucuronide) CHEMBL1697724 estradiol 17b glucuronide BDBM50344959 E17β-glucuronide
17beta-Estradiol-17-(beta-D-glucuronide) CHEMBL1697724 estradiol 17b glucuronide BDBM50344959 E17β-glucuronide BDBM50311133 17beta-[(N-Heptyl)methylamino]-4-methyl-4-aza-5alpha-androstan-3-one CHEMBL1080041
BDBM50311133 17beta-[(N-Heptyl)methylamino]-4-methyl-4-aza-5alpha-androstan-3-one CHEMBL1080041 CHEMBL1079512 17beta-[(N-Decyl)formamido]-4-methyl-4-aza-5alpha-androstan-3-one BDBM50311132
CHEMBL1079512 17beta-[(N-Decyl)formamido]-4-methyl-4-aza-5alpha-androstan-3-one BDBM50311132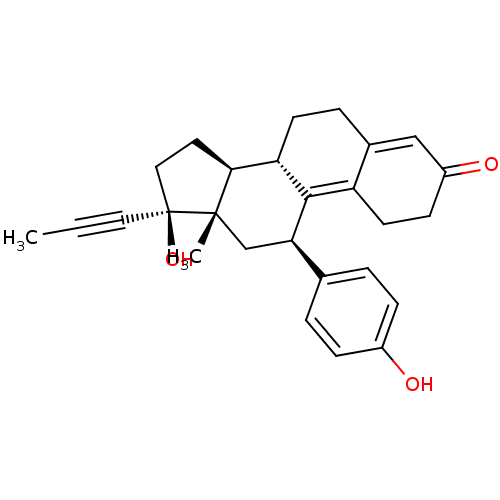 CHEMBL490087 BDBM50292751 17beta-Hydroxy-11beta-[4-hydroxyphenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
CHEMBL490087 BDBM50292751 17beta-Hydroxy-11beta-[4-hydroxyphenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one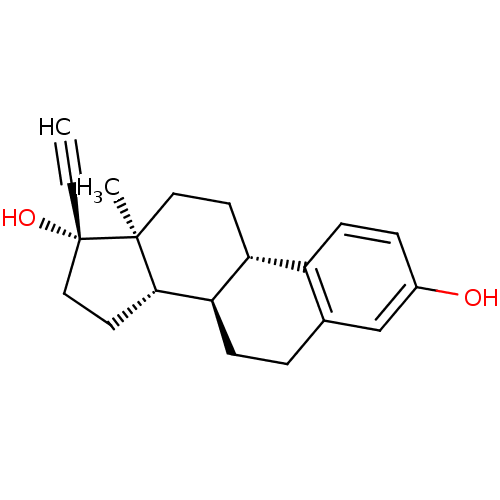 17-ethinyl-3,17-estradiol Ethinylestradiol CHEMBL691 17alpha-ethynylestra-1,3,5(10)-triene-3,17beta-diol ETHINYL ESTRADIOL Ethynyl estradiol 17alpha-Ethinyl estradiol 17alpha-ethynylestradiol 17-ethinyl-3,17-oestradiol ethinyloestradiol 17-ethinylestradiol BDBM50187243
17-ethinyl-3,17-estradiol Ethinylestradiol CHEMBL691 17alpha-ethynylestra-1,3,5(10)-triene-3,17beta-diol ETHINYL ESTRADIOL Ethynyl estradiol 17alpha-Ethinyl estradiol 17alpha-ethynylestradiol 17-ethinyl-3,17-oestradiol ethinyloestradiol 17-ethinylestradiol BDBM50187243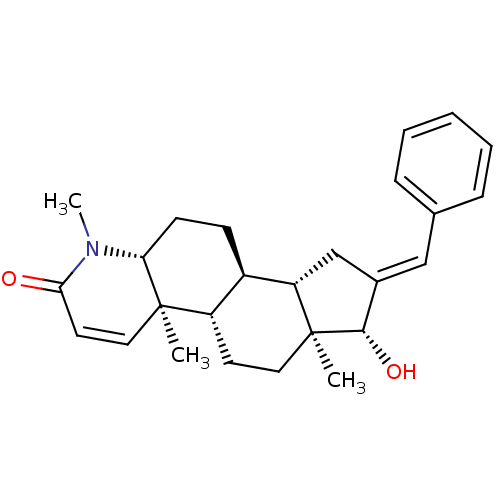 16-(Phenylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL560935 BDBM50296938
16-(Phenylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL560935 BDBM50296938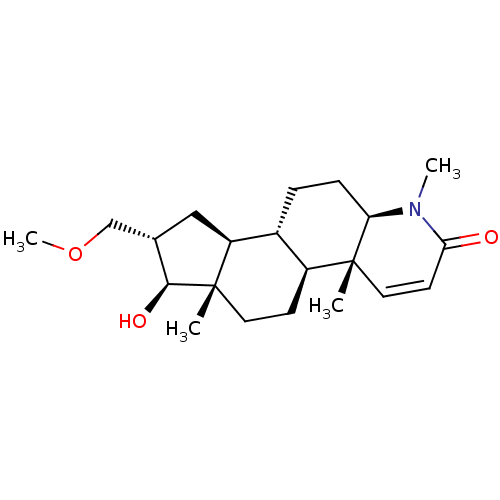 16alpha-(Methoxymethyl)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL538877 BDBM50296934
16alpha-(Methoxymethyl)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL538877 BDBM50296934 BDBM50296939 16-(Methylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL557116
BDBM50296939 16-(Methylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL557116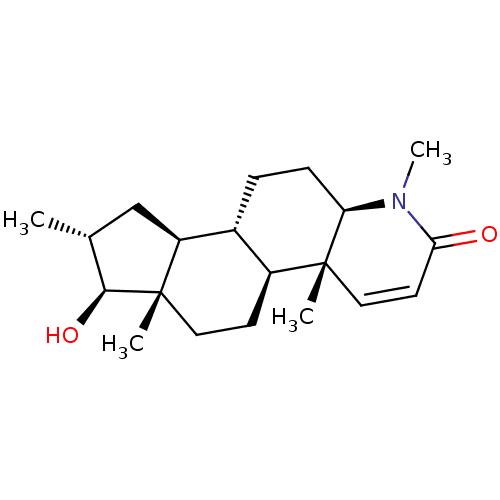 CHEMBL538137 16alpha-(Methyl)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296933
CHEMBL538137 16alpha-(Methyl)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296933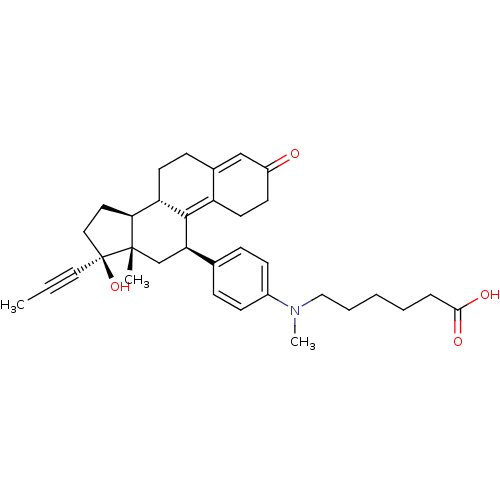 17beta-Hydroxy-11beta-[4-(5-carboxy-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292761 6-N-Methyl-N-[4'-[17beta-hydroxy-3-oxo-17alpha-(1-propynyl)-estra-4,9-dien-11beta-yl]-phenylaminohexanoicacid CHEMBL452860
17beta-Hydroxy-11beta-[4-(5-carboxy-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292761 6-N-Methyl-N-[4'-[17beta-hydroxy-3-oxo-17alpha-(1-propynyl)-estra-4,9-dien-11beta-yl]-phenylaminohexanoicacid CHEMBL452860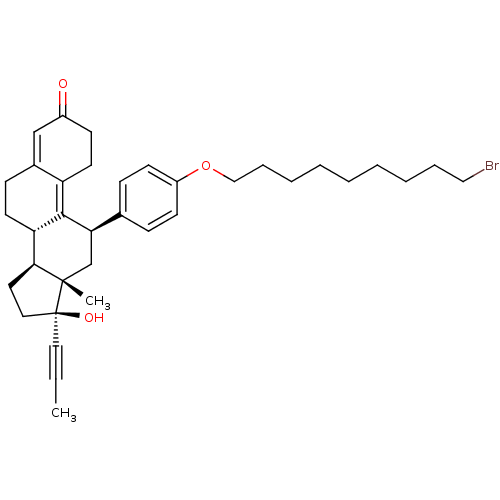 17beta-Hydroxy-11beta-[4-(9-bromnonoxy)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292752 CHEMBL454435
17beta-Hydroxy-11beta-[4-(9-bromnonoxy)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292752 CHEMBL454435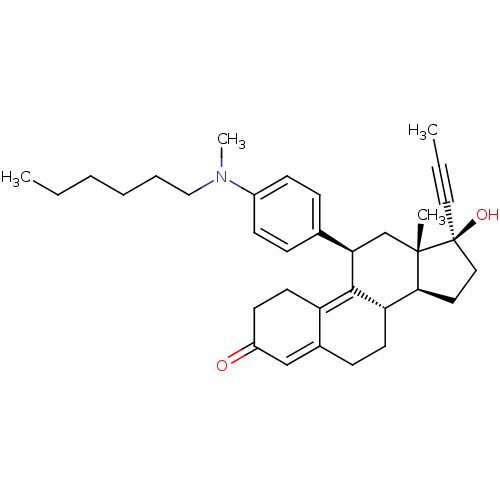 17beta-Hydroxy-11beta-[4-(N-methylhexylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292754 CHEMBL493845
17beta-Hydroxy-11beta-[4-(N-methylhexylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292754 CHEMBL493845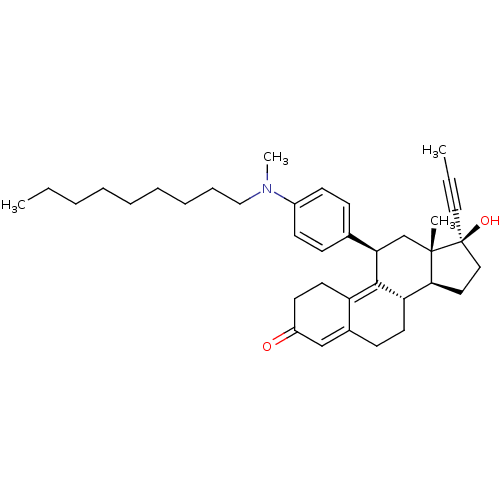 BDBM50292755 17beta-Hydroxy-11beta-[4-(N-methynonylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL459203
BDBM50292755 17beta-Hydroxy-11beta-[4-(N-methynonylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL459203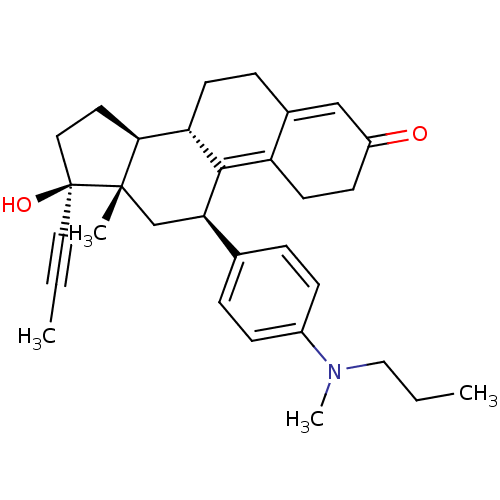 CHEMBL490088 BDBM50292753 17beta-Hydroxy-11beta-[4-(N-methylpropylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
CHEMBL490088 BDBM50292753 17beta-Hydroxy-11beta-[4-(N-methylpropylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL556229 BDBM50296935 16alpha-(3-Fluorobenzyl)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one
CHEMBL556229 BDBM50296935 16alpha-(3-Fluorobenzyl)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one 16-(Pyridin-3-ylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296942 CHEMBL562780
16-(Pyridin-3-ylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296942 CHEMBL562780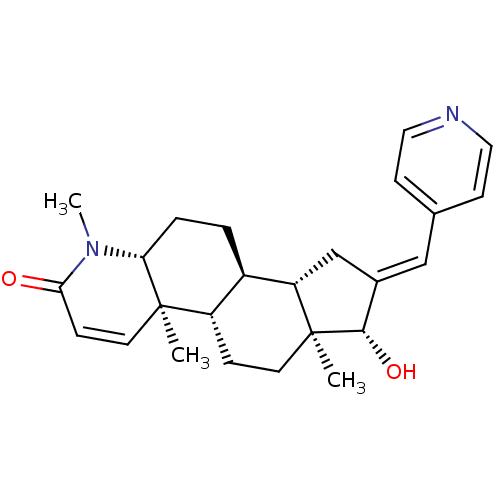 16-(Pyridin-4-ylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL556848 BDBM50296941
16-(Pyridin-4-ylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL556848 BDBM50296941 16-[(3,5-Difluorophenyl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL556431 BDBM50296937
16-[(3,5-Difluorophenyl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL556431 BDBM50296937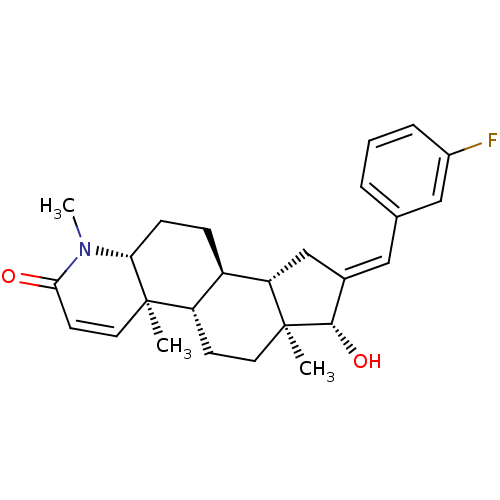 16-[(3-Fluorophenyl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296936 CHEMBL562471
16-[(3-Fluorophenyl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296936 CHEMBL562471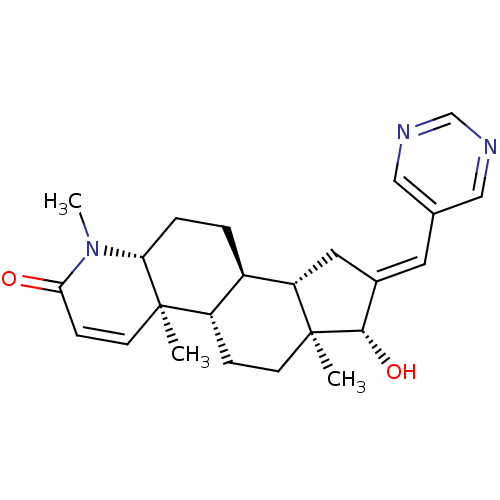 CHEMBL551269 16-(Pyrimidin-5-ylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296943
CHEMBL551269 16-(Pyrimidin-5-ylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296943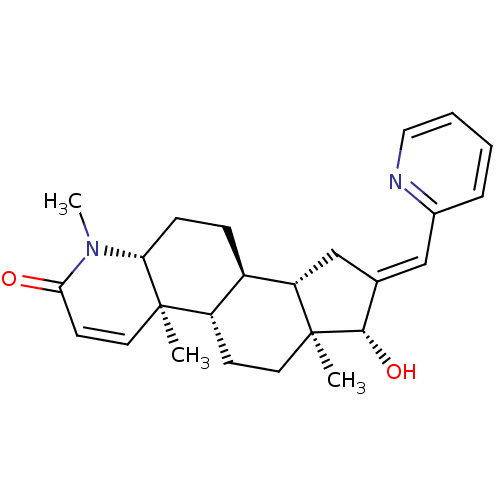 CHEMBL559485 BDBM50296940 16-(Pyridin-2-ylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one
CHEMBL559485 BDBM50296940 16-(Pyridin-2-ylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one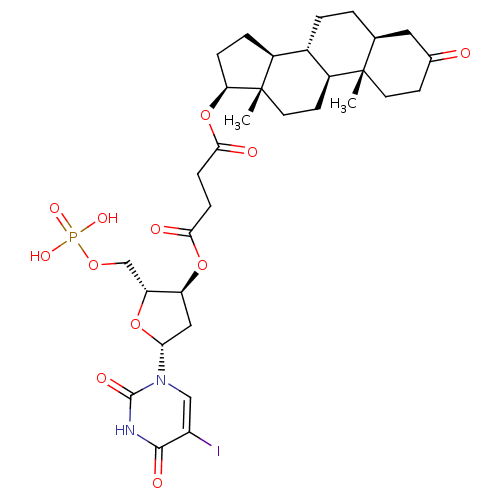 CHEMBL566118 5-[125I]Iodo-3'-O-(17beta-succinyl-5alpha-androstan-3-one)-2'-deoxyuridin-5'-yl Monophosphate BDBM50300786
CHEMBL566118 5-[125I]Iodo-3'-O-(17beta-succinyl-5alpha-androstan-3-one)-2'-deoxyuridin-5'-yl Monophosphate BDBM50300786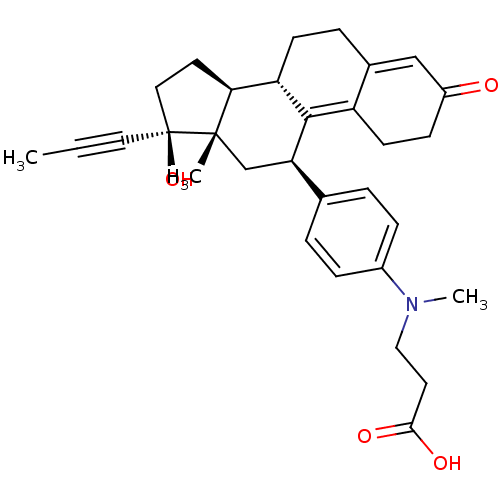 3-N-Methyl-N-[4-(11beta,17beta)-17-hydroxy-3-oxo-17alpha-(1-propynyl)estra-4,9-dien-11-yl]-phenylaminopropionic acid CHEMBL453382 BDBM50292760 17beta-Hydroxy-11beta-[4-(2-carboxy-N-methylethylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
3-N-Methyl-N-[4-(11beta,17beta)-17-hydroxy-3-oxo-17alpha-(1-propynyl)estra-4,9-dien-11-yl]-phenylaminopropionic acid CHEMBL453382 BDBM50292760 17beta-Hydroxy-11beta-[4-(2-carboxy-N-methylethylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one 17beta-Hydroxy-11beta-[4-(1-methyl-3-butylureido)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL453361 BDBM50292775
17beta-Hydroxy-11beta-[4-(1-methyl-3-butylureido)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL453361 BDBM50292775 17beta-Hydroxy-11beta-[4-(1-oxo-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292770 CHEMBL455176
17beta-Hydroxy-11beta-[4-(1-oxo-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292770 CHEMBL455176 17beta-Hydroxy-11beta-[4-(2-methoxycarbonyl-N-methylethylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292764 CHEMBL455175
17beta-Hydroxy-11beta-[4-(2-methoxycarbonyl-N-methylethylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292764 CHEMBL455175 17beta-Hydroxy-11beta-[4-(5-ethoxycarbonyl-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL504799 BDBM50292765
17beta-Hydroxy-11beta-[4-(5-ethoxycarbonyl-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL504799 BDBM50292765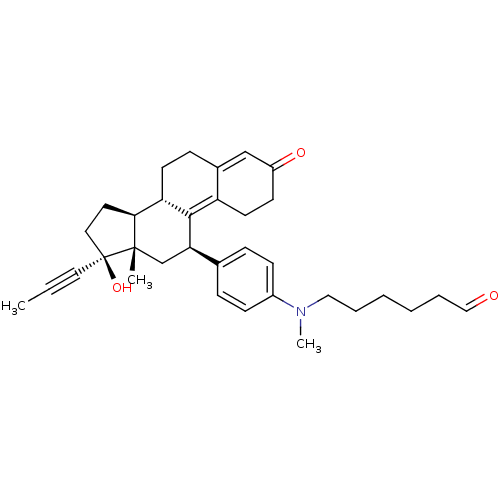 17beta-Hydroxy-11beta-[4-(5-formyl-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL453381 BDBM50292759
17beta-Hydroxy-11beta-[4-(5-formyl-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL453381 BDBM50292759 17beta-Hydroxy-11beta-[4-(7-carboxy-N-methylheptylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292762 CHEMBL510869
17beta-Hydroxy-11beta-[4-(7-carboxy-N-methylheptylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292762 CHEMBL510869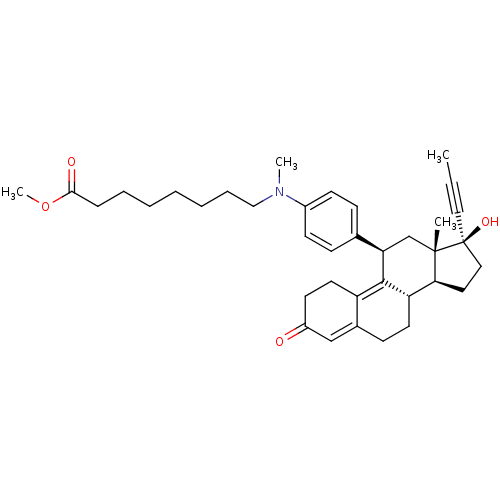 17beta-Hydroxy-11beta-[4-(7-methoxycarbonyl-N-methylheptylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292766 CHEMBL499452
17beta-Hydroxy-11beta-[4-(7-methoxycarbonyl-N-methylheptylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292766 CHEMBL499452 BDBM50197371 17beta-Hydroxy-7a-{5-(4,4,5,5,5-pentafluoropentylsulfinyl)17b-Hydroxy-7a-{5-(4,4,5,5,5-pentafluoropentylsulfinyl)pentyl}-5a-androstan-3-one CHEMBL224736
BDBM50197371 17beta-Hydroxy-7a-{5-(4,4,5,5,5-pentafluoropentylsulfinyl)17b-Hydroxy-7a-{5-(4,4,5,5,5-pentafluoropentylsulfinyl)pentyl}-5a-androstan-3-one CHEMBL224736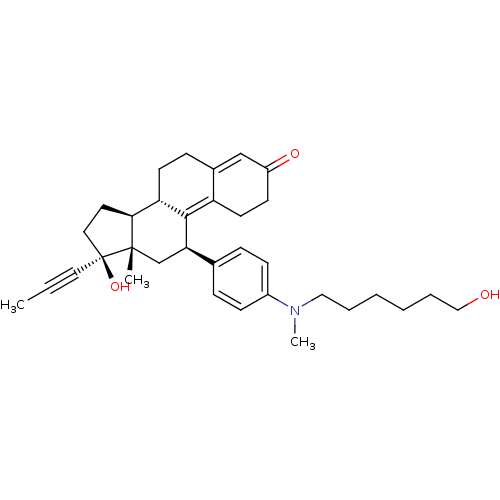 BDBM50292757 17beta-Hydroxy-11beta-[4-(6-hydroxy-N-methylhexylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL455411
BDBM50292757 17beta-Hydroxy-11beta-[4-(6-hydroxy-N-methylhexylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL455411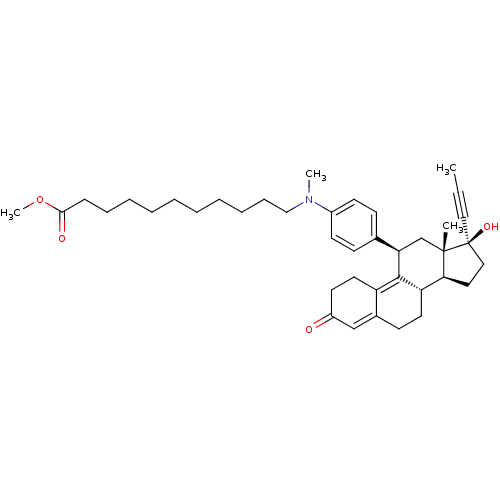 BDBM50292767 CHEMBL450052 17beta-Hydroxy-11beta-[4-(10-methoxycarbonyl-N-methyldecylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
BDBM50292767 CHEMBL450052 17beta-Hydroxy-11beta-[4-(10-methoxycarbonyl-N-methyldecylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one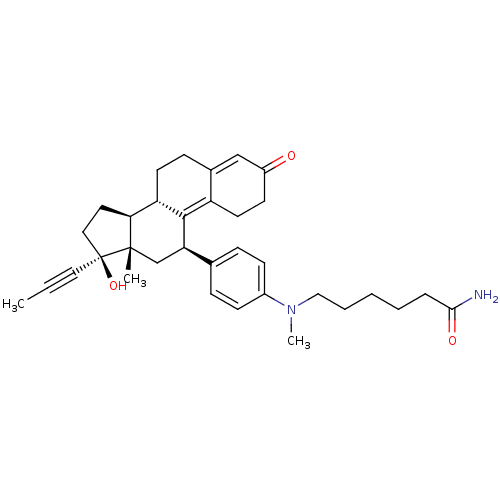 BDBM50292768 CHEMBL445043 17beta-Hydroxy-11beta-[4-(5-carbamoyl-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
BDBM50292768 CHEMBL445043 17beta-Hydroxy-11beta-[4-(5-carbamoyl-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL444027 BDBM50292763 17beta-Hydroxy-11beta-[4-(10-carboxy-N-methyldecylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
CHEMBL444027 BDBM50292763 17beta-Hydroxy-11beta-[4-(10-carboxy-N-methyldecylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one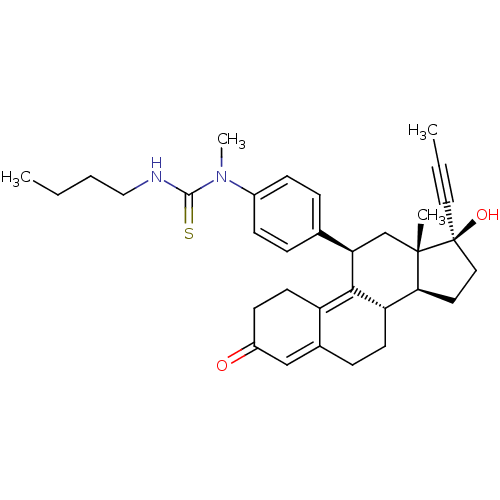 CHEMBL454167 BDBM50292776 17beta-Hydroxy-11beta-[4-(1-methyl-3-butylthioureido)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
CHEMBL454167 BDBM50292776 17beta-Hydroxy-11beta-[4-(1-methyl-3-butylthioureido)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one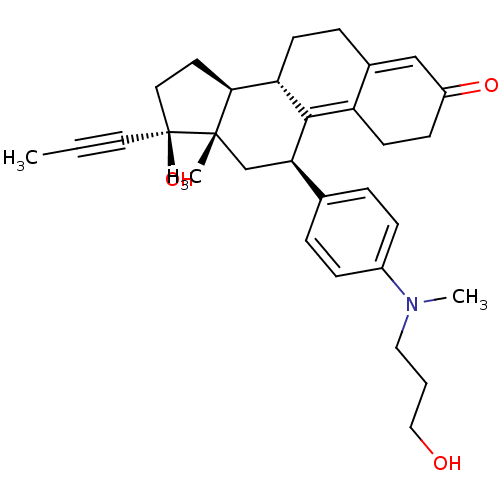 CHEMBL489300 BDBM50292756 17beta-Hydroxy-11beta-[4-(3-hydroxy-N-methypropylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
CHEMBL489300 BDBM50292756 17beta-Hydroxy-11beta-[4-(3-hydroxy-N-methypropylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one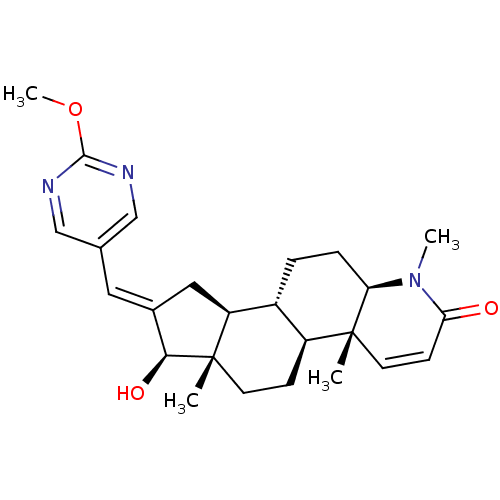 16-[(2-Methoxypyrimidin-5-yl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL559878 BDBM50296945
16-[(2-Methoxypyrimidin-5-yl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL559878 BDBM50296945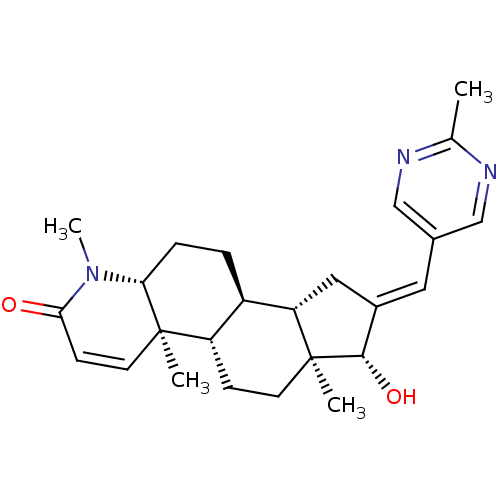 16-[(2-Methylpyrimidin-5-yl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296946 CHEMBL549375
16-[(2-Methylpyrimidin-5-yl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296946 CHEMBL549375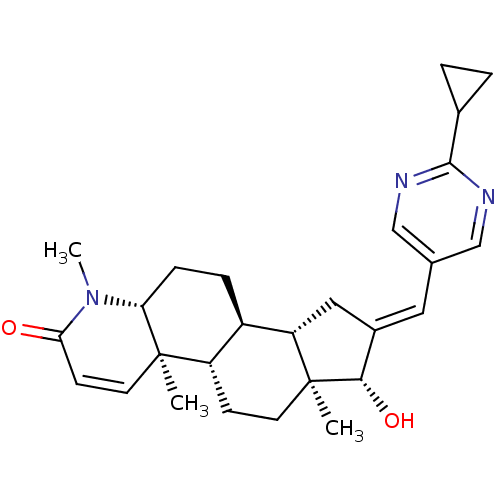 16-[(2-cyclopropylpyrimidin-5-yl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL551270 BDBM50296944
16-[(2-cyclopropylpyrimidin-5-yl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL551270 BDBM50296944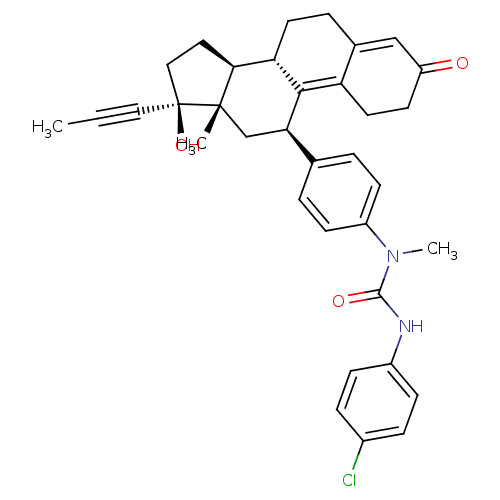 17beta-Hydroxy-11beta-{4-[methyl-3-(4-chlorphenyl)-ureido]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292777 CHEMBL449900
17beta-Hydroxy-11beta-{4-[methyl-3-(4-chlorphenyl)-ureido]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292777 CHEMBL449900 BDBM50292778 17beta-Hydroxy-11beta-{4-[methyl-3-(4-chlorphenyl)-thioureido]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL507916
BDBM50292778 17beta-Hydroxy-11beta-{4-[methyl-3-(4-chlorphenyl)-thioureido]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL507916 17beta-Hydroxy-11beta-[4-(1-oxo-4-methoxycarbonyl-N-methylbutylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292773 CHEMBL449367
17beta-Hydroxy-11beta-[4-(1-oxo-4-methoxycarbonyl-N-methylbutylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292773 CHEMBL449367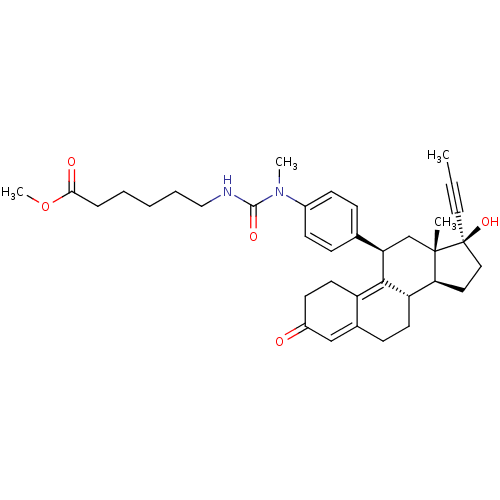 17beta-Hydroxy-11beta-{4-[1-methyl-3-(5-methoxycarbonylpentyl)-ureido]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292780 CHEMBL451127
17beta-Hydroxy-11beta-{4-[1-methyl-3-(5-methoxycarbonylpentyl)-ureido]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292780 CHEMBL451127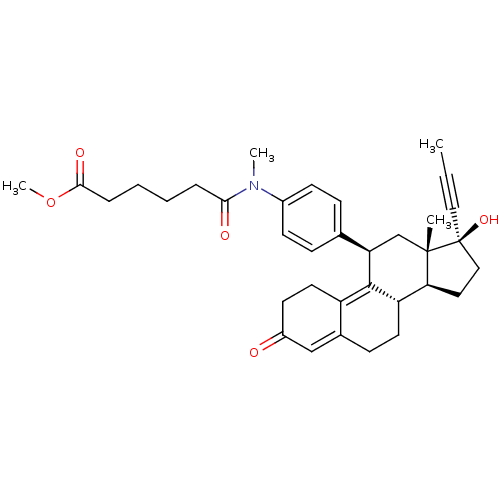 BDBM50292774 17beta-Hydroxy-11beta-[4-(1-oxo-5-methoxycarbonyl-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL454224
BDBM50292774 17beta-Hydroxy-11beta-[4-(1-oxo-5-methoxycarbonyl-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL454224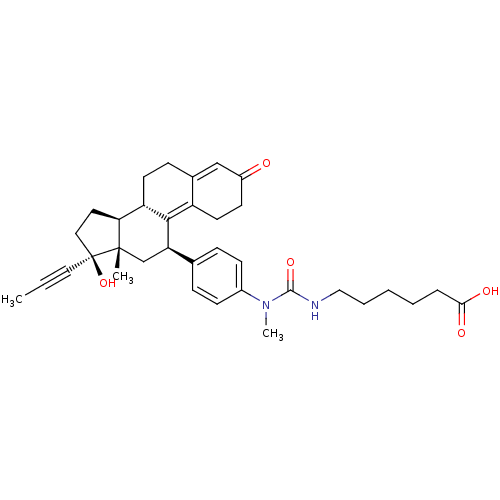 BDBM50292779 17beta-Hydroxy-11beta-{4-[1-methyl-3-(5-carboxypentyl)-ureido]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL503056
BDBM50292779 17beta-Hydroxy-11beta-{4-[1-methyl-3-(5-carboxypentyl)-ureido]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL503056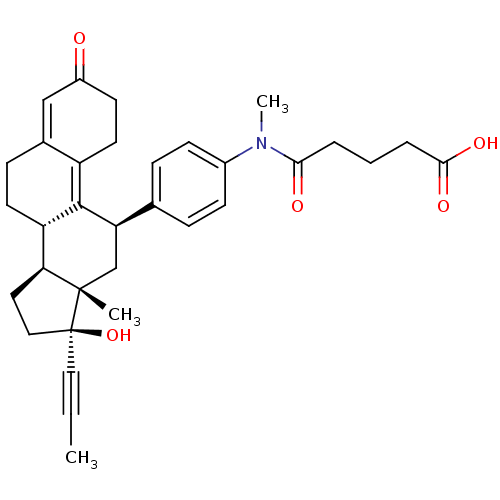 CHEMBL500992 17beta-Hydroxy-11beta-[4-(1-oxo-4-carboxy-N-methylbutylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292771
CHEMBL500992 17beta-Hydroxy-11beta-[4-(1-oxo-4-carboxy-N-methylbutylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292771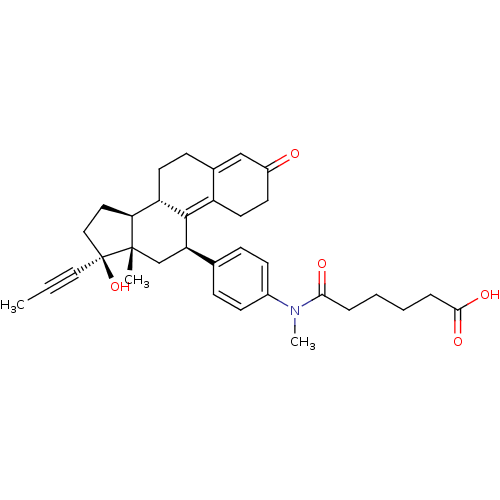 CHEMBL505267 BDBM50292772 17beta-Hydroxy-11beta-[4-(1-oxo-5-carboxy-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
CHEMBL505267 BDBM50292772 17beta-Hydroxy-11beta-[4-(1-oxo-5-carboxy-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL563597 16-{[(2-Methylamino)pyrimidin-5-yl]methylidene}-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296947
CHEMBL563597 16-{[(2-Methylamino)pyrimidin-5-yl]methylidene}-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296947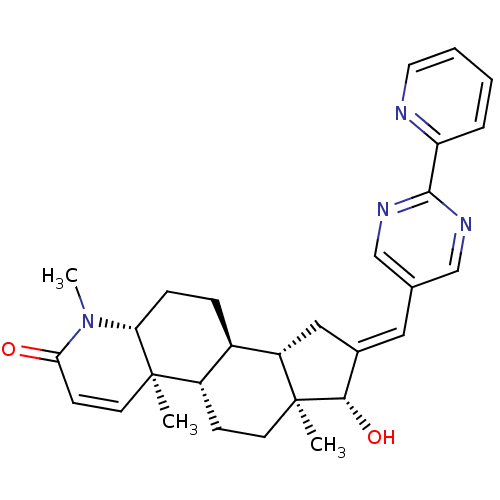 16-{[(Pyridin-2-yl)pyrimidin-5-yl]methylidene}-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL562394 BDBM50296948
16-{[(Pyridin-2-yl)pyrimidin-5-yl]methylidene}-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL562394 BDBM50296948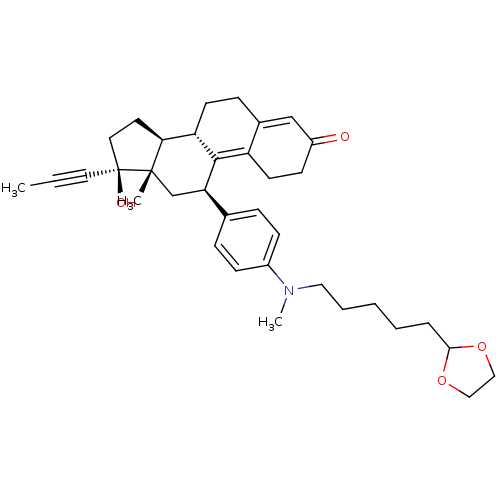 BDBM50292758 CHEMBL507253 17beta-Hydroxy-11beta-{4-[5-(1,3-dioxol-2-yl)-N-methylpentylamino]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one
BDBM50292758 CHEMBL507253 17beta-Hydroxy-11beta-{4-[5-(1,3-dioxol-2-yl)-N-methylpentylamino]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one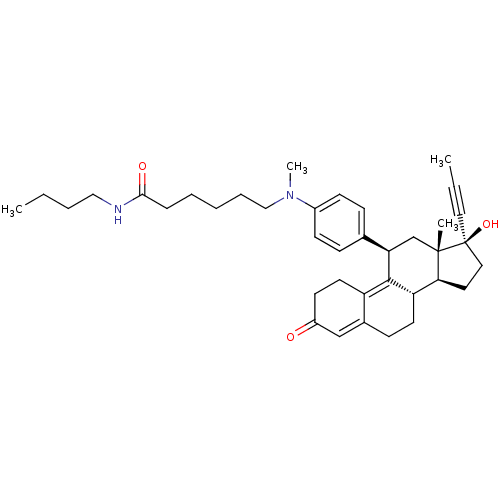 BDBM50292769 17beta-Hydroxy-11beta-[4-(1-methyl-7-oxo-1,8-diaza-dodecyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL505959
BDBM50292769 17beta-Hydroxy-11beta-[4-(1-methyl-7-oxo-1,8-diaza-dodecyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL505959 17beta-Hydroxy-11beta-[4-(1-methyl-6,9-dioxo-1-aza-5,10-dioxa-undecyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292787 CHEMBL506443
17beta-Hydroxy-11beta-[4-(1-methyl-6,9-dioxo-1-aza-5,10-dioxa-undecyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292787 CHEMBL506443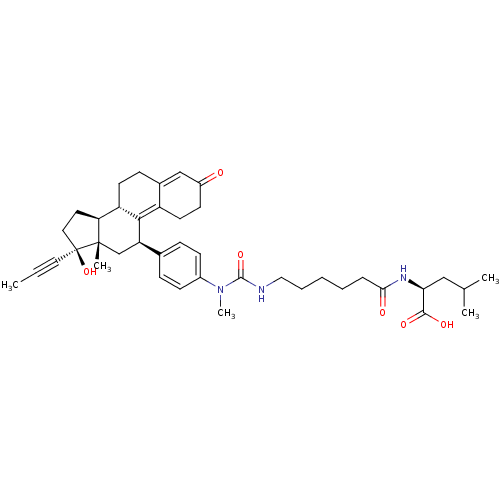 BDBM50292782 CHEMBL461357 17beta-Hydroxy-11beta-{4-[11-carboxy-1,13-dimethyl-2,9-dioxo-1,3,10-triaza-tetradecyl]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one
BDBM50292782 CHEMBL461357 17beta-Hydroxy-11beta-{4-[11-carboxy-1,13-dimethyl-2,9-dioxo-1,3,10-triaza-tetradecyl]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one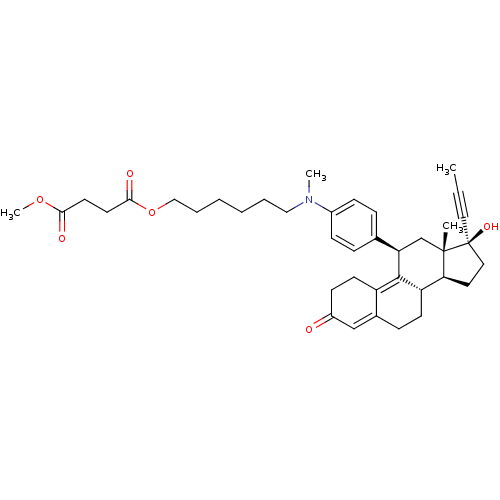 BDBM50292788 17beta-Hydroxy-11beta-[4-(1-methyl-9,12-dioxo-1-aza-8,13-dioxa-tetradecyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL455530
BDBM50292788 17beta-Hydroxy-11beta-[4-(1-methyl-9,12-dioxo-1-aza-8,13-dioxa-tetradecyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL455530 17beta-estradiol (E2) 17α-ethinylestradiol Ovocyclin US9561238, E2 [2,4,6,7-3H]-17beta-estradiol CHEMBL135 (1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2,4,6-triene-5,14-diol US9034854, E2 CS336 [3H]]estradiol [2,4,6,7-3H]-E2 ESTRADIOL US9422324, E2 BDBM17292 [3H]-estradiol US9040509, E2 17 beta-Estradiol Estradiol-17 alpha
17beta-estradiol (E2) 17α-ethinylestradiol Ovocyclin US9561238, E2 [2,4,6,7-3H]-17beta-estradiol CHEMBL135 (1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2,4,6-triene-5,14-diol US9034854, E2 CS336 [3H]]estradiol [2,4,6,7-3H]-E2 ESTRADIOL US9422324, E2 BDBM17292 [3H]-estradiol US9040509, E2 17 beta-Estradiol Estradiol-17 alpha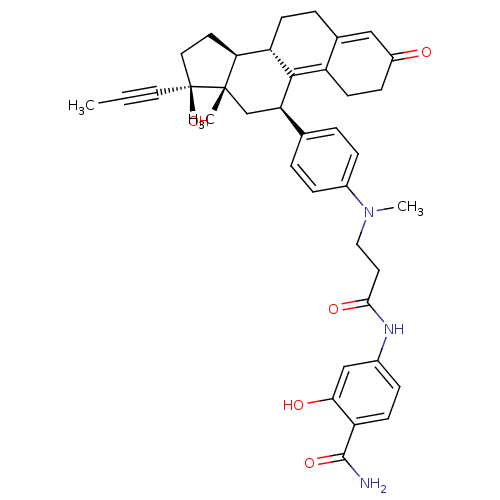 17beta-Hydroxy-11beta-{4-[1-methyl-4-oxo-5-(4-carbamoyl-3-hydroxyphenyl)-1,5-diazapentyl]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292783 CHEMBL509832
17beta-Hydroxy-11beta-{4-[1-methyl-4-oxo-5-(4-carbamoyl-3-hydroxyphenyl)-1,5-diazapentyl]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292783 CHEMBL509832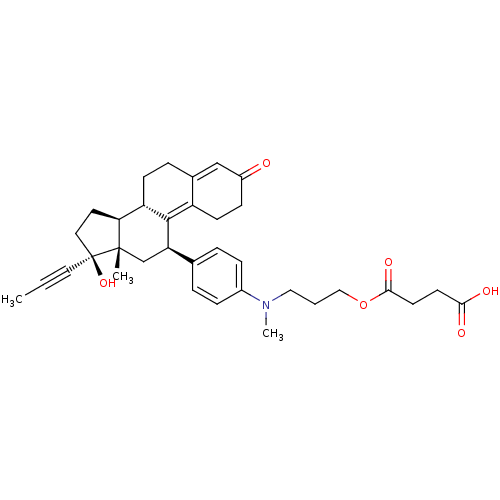 CHEMBL446933 BDBM50292785 17beta-Hydroxy-11beta-[4-(9-hydroxy-1-methyl-6,9-dioxo-1-aza-5-oxa-nonyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
CHEMBL446933 BDBM50292785 17beta-Hydroxy-11beta-[4-(9-hydroxy-1-methyl-6,9-dioxo-1-aza-5-oxa-nonyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one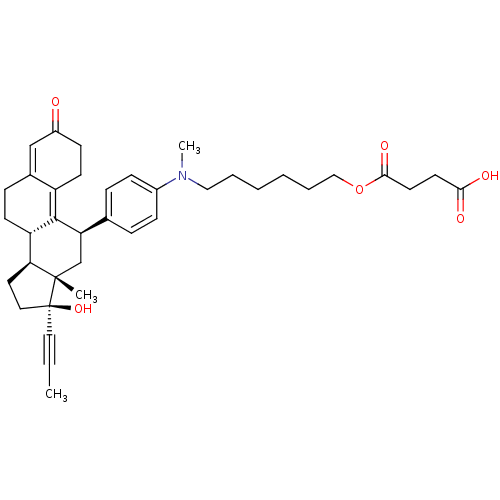 CHEMBL451680 BDBM50292786 17beta-Hydroxy-11beta-[4-(12-hydroxy-1-methyl-9,12-dioxo-1-aza-8-oxa-dodecyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
CHEMBL451680 BDBM50292786 17beta-Hydroxy-11beta-[4-(12-hydroxy-1-methyl-9,12-dioxo-1-aza-8-oxa-dodecyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292784 CHEMBL504252 17beta-Hydroxy-11beta-{4-[1-methyl-6-(4-carbamoyl-3-hydroxyphenyl)-1-aza-5-oxa-hexyl]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one
BDBM50292784 CHEMBL504252 17beta-Hydroxy-11beta-{4-[1-methyl-6-(4-carbamoyl-3-hydroxyphenyl)-1-aza-5-oxa-hexyl]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one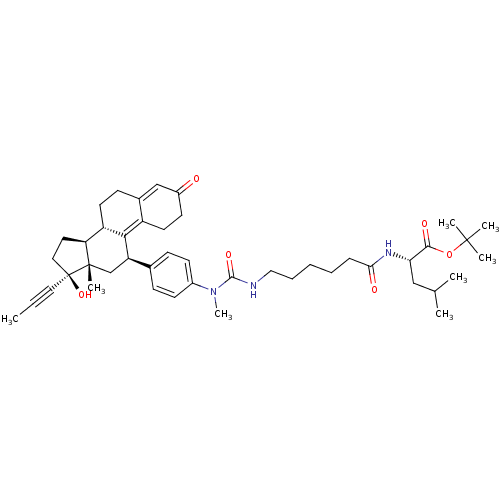 CHEMBL454121 BDBM50292781 17beta-Hydroxy-11beta-{4-[1,14,14-trimethyl-11-(2-methylpropyl)-2,9,12-trioxo-1,3,10-triaza-13-oxa-pentadecyl]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one
CHEMBL454121 BDBM50292781 17beta-Hydroxy-11beta-{4-[1,14,14-trimethyl-11-(2-methylpropyl)-2,9,12-trioxo-1,3,10-triaza-13-oxa-pentadecyl]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one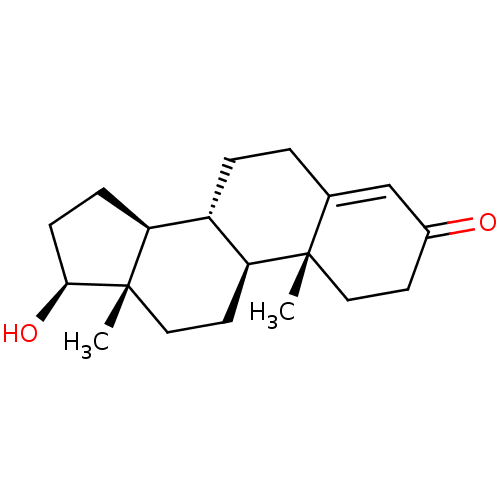 (1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one Testosterone US9682960, Testosterone Testosterone, 1 BDBM8885 17beta-Hydroxyandrost-4-en-3-one
(1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one Testosterone US9682960, Testosterone Testosterone, 1 BDBM8885 17beta-Hydroxyandrost-4-en-3-one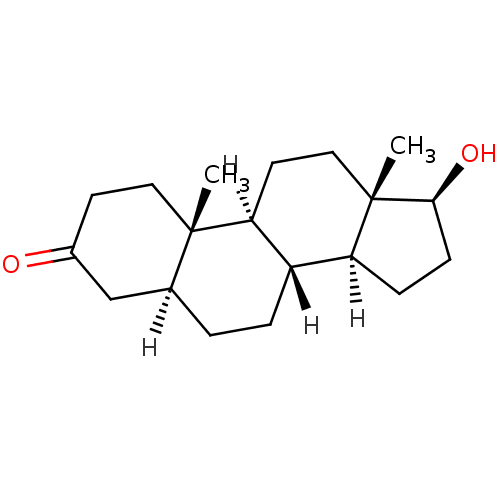 DHT Dihydrotestosterone [3H]DHT (5alpha,17beta)-17-hydroxyandrostan-3-one BDBM50366473 (1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-5-one BDBM18161 CHEMBL27769
DHT Dihydrotestosterone [3H]DHT (5alpha,17beta)-17-hydroxyandrostan-3-one BDBM50366473 (1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-5-one BDBM18161 CHEMBL27769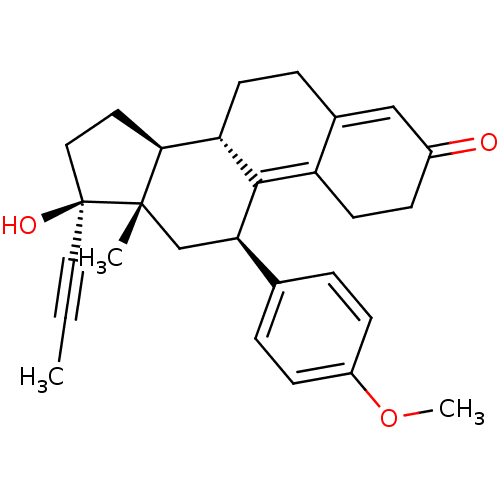 17beta-Hydroxy-11beta-[4-methoxyphenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one (8S,11R,13S,14S,17S)-17-hydroxy-11-(4-methoxy-phenyl)-13-methyl-17-prop-1-ynyl-1,2,6,7,8,11,12,13,14,15,16,17-dodecahydro-cyclopenta[a]phenanthren-3-one CHEMBL221686 BDBM50195153
17beta-Hydroxy-11beta-[4-methoxyphenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one (8S,11R,13S,14S,17S)-17-hydroxy-11-(4-methoxy-phenyl)-13-methyl-17-prop-1-ynyl-1,2,6,7,8,11,12,13,14,15,16,17-dodecahydro-cyclopenta[a]phenanthren-3-one CHEMBL221686 BDBM50195153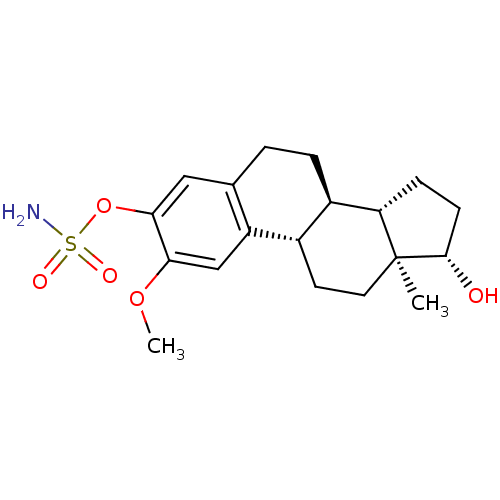 BDBM50171448 CHEMBL190628 Sulfamic acid (11R,12S,15S,16S)-17-(S)-hydroxy-2-methoxy-13-methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-3-yl ester (9BETA,14BETA,17BETA)-17-HYDROXY-2-METHOXYESTRA-1,3,5(10)-TRIEN-3-YL SULFAMATE 2-methoxyestradiol-3-O-sulfamate
BDBM50171448 CHEMBL190628 Sulfamic acid (11R,12S,15S,16S)-17-(S)-hydroxy-2-methoxy-13-methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-3-yl ester (9BETA,14BETA,17BETA)-17-HYDROXY-2-METHOXYESTRA-1,3,5(10)-TRIEN-3-YL SULFAMATE 2-methoxyestradiol-3-O-sulfamate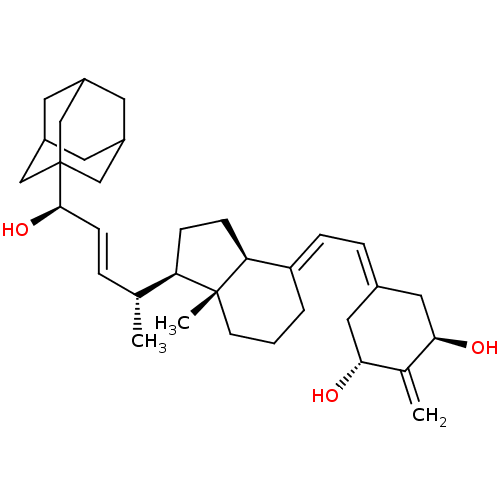 CHEMBL467212 (24R)-24-Adamantyl-1r,24-dihydroxy-2-methylene-22,23-didehydro-19,25,26,27-tetranorvitamin D3 BDBM50292706 (1R,3R,7E,17beta)-17-{(1R,2E,4R)-4-hydroxy-1-methyl-4-[(3S,5S,7S)-tricyclo[3.3.1.1~3,7~]dec-1-yl]but-2-en-1-yl}-2-methylidene-9,10-secoestra-5,7-diene-1,3-diol
CHEMBL467212 (24R)-24-Adamantyl-1r,24-dihydroxy-2-methylene-22,23-didehydro-19,25,26,27-tetranorvitamin D3 BDBM50292706 (1R,3R,7E,17beta)-17-{(1R,2E,4R)-4-hydroxy-1-methyl-4-[(3S,5S,7S)-tricyclo[3.3.1.1~3,7~]dec-1-yl]but-2-en-1-yl}-2-methylidene-9,10-secoestra-5,7-diene-1,3-diol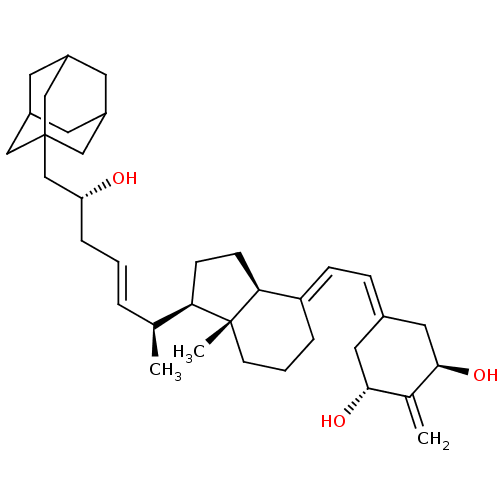 (1R,3R,7E,17beta)-17-{(1S,2E,5R)-5-hydroxy-1-methyl-6-[(3S,5S,7S)-tricyclo[3.3.1.1~3,7~]dec-1-yl]hex-2-en-1-yl}-2-methylidene-9,10-secoestra-5,7-diene-1,3-diol CHEMBL452878 (25R)-26-adamantyl-1R,25-hydroxy-2-methylene-22,23-didehydro-19,27-dinor-20-epivitamin D3 BDBM50292704
(1R,3R,7E,17beta)-17-{(1S,2E,5R)-5-hydroxy-1-methyl-6-[(3S,5S,7S)-tricyclo[3.3.1.1~3,7~]dec-1-yl]hex-2-en-1-yl}-2-methylidene-9,10-secoestra-5,7-diene-1,3-diol CHEMBL452878 (25R)-26-adamantyl-1R,25-hydroxy-2-methylene-22,23-didehydro-19,27-dinor-20-epivitamin D3 BDBM50292704 [(8R,9S,13S,14S,17S)-3-hydroxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl] acetate acetic acid [(8R,9S,13S,14S,17S)-3-hydroxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl] ester [(8R,9S,13S,14S,17S)-13-methyl-3-oxidanyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl] ethanoate BDBM62878 cid_6852404 MLS000069774 SMR000058696 17BETA-ESTRADIOL 17-ACETATE
[(8R,9S,13S,14S,17S)-3-hydroxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl] acetate acetic acid [(8R,9S,13S,14S,17S)-3-hydroxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl] ester [(8R,9S,13S,14S,17S)-13-methyl-3-oxidanyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl] ethanoate BDBM62878 cid_6852404 MLS000069774 SMR000058696 17BETA-ESTRADIOL 17-ACETATE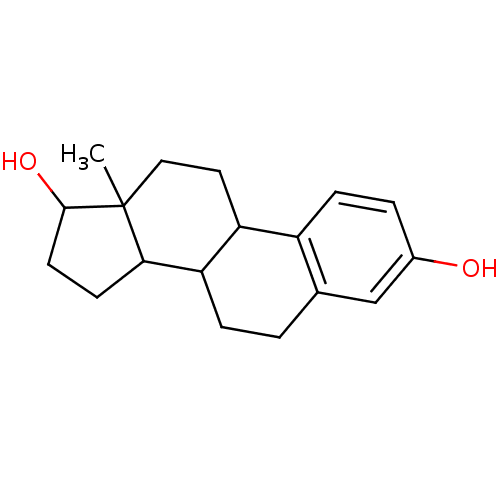 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol(17beta-estradiol) (estradiol)13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol([6,7-3H]-estradiol) 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol(estradiol) CHEMBL135 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol(17alpha-estradiol) 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol 17beta-Estradiol 1,5-Dimethyl-2-phenyl-1,2-dihydro-pyrazol-3-one BDBM50005414 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol (estradiol) ESTRADIOL 15-methyltetracyclo[8.7.0.02,7.011,15]heptadeca-2,4,6-triene-5,14-diol Estradiol13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol( Estradiol)
13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol(17beta-estradiol) (estradiol)13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol([6,7-3H]-estradiol) 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol(estradiol) CHEMBL135 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol(17alpha-estradiol) 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol 17beta-Estradiol 1,5-Dimethyl-2-phenyl-1,2-dihydro-pyrazol-3-one BDBM50005414 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol (estradiol) ESTRADIOL 15-methyltetracyclo[8.7.0.02,7.011,15]heptadeca-2,4,6-triene-5,14-diol Estradiol13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol( Estradiol)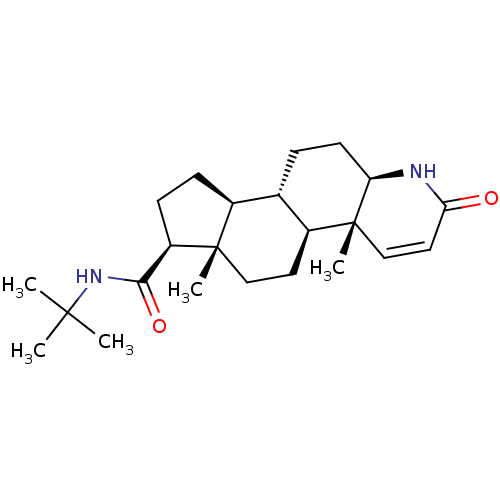 Proscar BDBM50334788 (4aR,6aS,7S)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide CHEMBL710 FINASTERIDE 4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide(Finasteride) MK-906 (R)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (4aR,4bS,6aS,7S,9aS,9bS,11aR)-N-tert-butyl-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxamide Propecia (4aR,6aS,7S,11aR)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide US9061023, Finasteride (4aR,4bS,6aS,7S,9aS,9bS,11aR)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (4aR,4bS,6aS,9aS,9bS,11aR)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (4aR,6aS,11aR)-4a,6a-Dimethyl-2-oxo-hexadecahydro-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide 4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-androst-1-en-3-one 4a,6a,9a-Trimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid (Finasteride)
Proscar BDBM50334788 (4aR,6aS,7S)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide CHEMBL710 FINASTERIDE 4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide(Finasteride) MK-906 (R)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (4aR,4bS,6aS,7S,9aS,9bS,11aR)-N-tert-butyl-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxamide Propecia (4aR,6aS,7S,11aR)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide US9061023, Finasteride (4aR,4bS,6aS,7S,9aS,9bS,11aR)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (4aR,4bS,6aS,9aS,9bS,11aR)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (4aR,6aS,11aR)-4a,6a-Dimethyl-2-oxo-hexadecahydro-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide 4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-androst-1-en-3-one 4a,6a,9a-Trimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid (Finasteride)
- Vinh, TK; Nicholls, PJ; Kirby, AJ; Simons, C Evaluation of 7-hydroxy-flavones as inhibitors of oestrone and oestradiol biosynthesis. J Enzym Inhib 16: 417-24 (2001)
- Le Lain, R; Nicholls, PJ; Smith, HJ; Maharlouie, FH Inhibitors of human and rat testes microsomal 17beta-hydroxysteroid dehydrogenase (17beta-HSD) as potential agents for prostatic cancer. J Enzym Inhib 16: 35-45 (2001)
- Sugiyama, D; Kusuhara, H; Shitara, Y; Abe, T; Meier, PJ; Sekine, T; Endou, H; Suzuki, H; Sugiyama, Y Characterization of the efflux transport of 17beta-estradiol-D-17beta-glucuronide from the brain across the blood-brain barrier. J Pharmacol Exp Ther 298: 316-22 (2001)
- Hu, GX; Liang, G; Chu, Y; Li, X; Lian, QQ; Lin, H; He, Y; Huang, Y; Hardy, DO; Ge, RS Curcumin derivatives inhibit testicular 17beta-hydroxysteroid dehydrogenase 3. Bioorg Med Chem Lett 20: 2549-51 (2010)
- Lawrence, HR; Vicker, N; Allan, GM; Smith, A; Mahon, MF; Tutill, HJ; Purohit, A; Reed, MJ; Potter, BV Novel and potent 17beta-hydroxysteroid dehydrogenase type 1 inhibitors. J Med Chem 48: 2759-62 (2005)
- Oster, A; Klein, T; Werth, R; Kruchten, P; Bey, E; Negri, M; Marchais-Oberwinkler, S; Frotscher, M; Hartmann, RW Novel estrone mimetics with high 17beta-HSD1 inhibitory activity. Bioorg Med Chem 18: 3494-505 (2010)
- Procopiou, PA; Biggadike, K; English, AF; Farrell, RM; Hagger, GN; Hancock, AP; Haase, MV; Irving, WR; Sareen, M; Snowden, MA; Solanke, YE; Tralau-Stewart, CJ; Walton, SE; Wood, JA Novel glucocorticoid antedrugs possessing a 17beta-(gamma-lactone) ring. J Med Chem 44: 602-12 (2001)
- Marchais-Oberwinkler, S; Kruchten, P; Frotscher, M; Ziegler, E; Neugebauer, A; Bhoga, U; Bey, E; Müller-Vieira, U; Messinger, J; Thole, H; Hartmann, RW Substituted 6-phenyl-2-naphthols. Potent and selective nonsteroidal inhibitors of 17beta-hydroxysteroid dehydrogenase type 1 (17beta-HSD1): design, synthesis, biological evaluation, and pharmacokinetics. J Med Chem 51: 4685-98 (2008)
- Lilienkampf, A; Karkola, S; Alho-Richmond, S; Koskimies, P; Johansson, N; Huhtinen, K; Vihko, K; Wähälä, K Synthesis and biological evaluation of 17beta-hydroxysteroid dehydrogenase type 1 (17beta-HSD1) inhibitors based on a thieno[2,3-d]pyrimidin-4(3H)-one core. J Med Chem 52: 6660-71 (2009)
- Allan, GM; Vicker, N; Lawrence, HR; Tutill, HJ; Day, JM; Huchet, M; Ferrandis, E; Reed, MJ; Purohit, A; Potter, BV Novel inhibitors of 17beta-hydroxysteroid dehydrogenase type 1: templates for design. Bioorg Med Chem 16: 4438-56 (2008)
- Lota, RK; Dhanani, S; Owen, CP; Ahmed, S Synthesis, biochemical evaluation and rationalisation of the inhibitory activity of a series of 4-hydroxyphenyl ketones as potential inhibitors of 17beta-hydroxysteroid dehydrogenase type 3 (17beta-HSD3). Bioorg Med Chem Lett 16: 4519-22 (2006)
- Ngatcha, BT; Luu-The, V; Poirier, D Androsterone 3beta-substituted derivatives as inhibitors of type 3 17beta-hydroxysteroid dehydrogenase. Bioorg Med Chem Lett 10: 2533-6 (2001)
- Poirier, D; Boivin, RP; Tremblay, MR; Bérubé, M; Qiu, W; Lin, SX Estradiol-adenosine hybrid compounds designed to inhibit type 1 17beta-hydroxysteroid dehydrogenase. J Med Chem 48: 8134-47 (2005)
- Hartmann, R; Frotscher, M; Oberwinkler, S; Ziegler, E; Messinger, J; Thole, H 17Beta-hydroxysteroid dehydrogenase type 1 inhibitors for the treatment of hormone-related diseases US Patent US8546392 (2013)
- Bey, E; Marchais-Oberwinkler, S; Werth, R; Negri, M; Al-Soud, YA; Kruchten, P; Oster, A; Frotscher, M; Birk, B; Hartmann, RW Design, synthesis, biological evaluation and pharmacokinetics of bis(hydroxyphenyl) substituted azoles, thiophenes, benzenes, and aza-benzenes as potent and selective nonsteroidal inhibitors of 17beta-hydroxysteroid dehydrogenase type 1 (17beta-HSD1). J Med Chem 51: 6725-39 (2008)
- Bey, E; Marchais-Oberwinkler, S; Negri, M; Kruchten, P; Oster, A; Klein, T; Spadaro, A; Werth, R; Frotscher, M; Birk, B; Hartmann, RW New insights into the SAR and binding modes of bis(hydroxyphenyl)thiophenes and -benzenes: influence of additional substituents on 17beta-hydroxysteroid dehydrogenase type 1 (17beta-HSD1) inhibitory activity and selectivity. J Med Chem 52: 6724-43 (2009)
- Bey, E; Marchais-Oberwinkler, S; Kruchten, P; Frotscher, M; Werth, R; Oster, A; Algül, O; Neugebauer, A; Hartmann, RW Design, synthesis and biological evaluation of bis(hydroxyphenyl) azoles as potent and selective non-steroidal inhibitors of 17beta-hydroxysteroid dehydrogenase type 1 (17beta-HSD1) for the treatment of estrogen-dependent diseases. Bioorg Med Chem 16: 6423-35 (2008)
- Frotscher, M; Ziegler, E; Marchais-Oberwinkler, S; Kruchten, P; Neugebauer, A; Fetzer, L; Scherer, C; Müller-Vieira, U; Messinger, J; Thole, H; Hartmann, RW Design, synthesis, and biological evaluation of (hydroxyphenyl)naphthalene and -quinoline derivatives: potent and selective nonsteroidal inhibitors of 17beta-hydroxysteroid dehydrogenase type 1 (17beta-HSD1) for the treatment of estrogen-dependent diseases. J Med Chem 51: 2158-69 (2008)
- Fischer, DS; Allan, GM; Bubert, C; Vicker, N; Smith, A; Tutill, HJ; Purohit, A; Wood, L; Packham, G; Mahon, MF; Reed, MJ; Potter, BV E-ring modified steroids as novel potent inhibitors of 17beta-hydroxysteroid dehydrogenase type 1. J Med Chem 48: 5749-70 (2005)
- Gunn, D; Akuche, C; Baryza, J; Blue, ML; Brennan, C; Campbell, AM; Choi, S; Cook, J; Conrad, P; Dixon, B; Dumas, J; Ehrlich, P; Gane, T; Joe, T; Johnson, J; Jordan, J; Kramss, R; Liu, P; Levy, J; Lowe, D; McAlexander, I; Natero, R; Redman, AM; Scott, W; Seng, T; Sibley, R; Wang, M; Wang, Y; Wood, J; Zhang, Z 4,5-Disubstituted cis-pyrrolidinones as inhibitors of type II 17beta-hydroxysteroid dehydrogenase. Part 2. SAR. Bioorg Med Chem Lett 15: 3053-7 (2005)
- Harada, K; Kubo, H; Tomigahara, Y; Nishioka, K; Takahashi, J; Momose, M; Inoue, S; Kojima, A Coumarins as novel 17beta-hydroxysteroid dehydrogenase type 3 inhibitors for potential treatment of prostate cancer. Bioorg Med Chem Lett 20: 272-5 (2010)
- Fournier, D; Poirier, D; Mazumdar, M; Lin, SX Design and synthesis of bisubstrate inhibitors of type 1 17beta-hydroxysteroid dehydrogenase: overview and perspectives. Eur J Med Chem 43: 2298-306 (2008)
- Fink, BE; Gavai, AV; Tokarski, JS; Goyal, B; Misra, R; Xiao, HY; Kimball, SD; Han, WC; Norris, D; Spires, TE; You, D; Gottardis, MM; Lorenzi, MV; Vite, GD Identification of a novel series of tetrahydrodibenzazocines as inhibitors of 17beta-hydroxysteroid dehydrogenase type 3. Bioorg Med Chem Lett 16: 1532-6 (2006)
- Schuster, D; Nashev, LG; Kirchmair, J; Laggner, C; Wolber, G; Langer, T; Odermatt, A Discovery of nonsteroidal 17beta-hydroxysteroid dehydrogenase 1 inhibitors by pharmacophore-based screening of virtual compound libraries. J Med Chem 51: 4188-99 (2008)
- Qiu, W; Campbell, RL; Gangloff, A; Dupuis, P; Boivin, RP; Tremblay, MR; Poirier, D; Lin, SX A concerted, rational design of type 1 17beta-hydroxysteroid dehydrogenase inhibitors: estradiol-adenosine hybrids with high affinity. FASEB J 16: 1829-31 (2002)
- Wood, J; Bagi, CM; Akuche, C; Bacchiocchi, A; Baryza, J; Blue, ML; Brennan, C; Campbell, AM; Choi, S; Cook, JH; Conrad, P; Dixon, BR; Ehrlich, PP; Gane, T; Gunn, D; Joe, T; Johnson, JS; Jordan, J; Kramss, R; Liu, P; Levy, J; Lowe, DB; McAlexander, I; Natero, R; Redman, AM; Scott, WJ; Town, C; Wang, M; Wang, Y; Zhang, Z 4,5-Disubstituted cis-pyrrolidinones as inhibitors of type II 17beta-hydroxysteroid dehydrogenase. Part 3. Identification of lead candidate. Bioorg Med Chem Lett 16: 4965-8 (2006)
- Allan, GM; Lawrence, HR; Cornet, J; Bubert, C; Fischer, DS; Vicker, N; Smith, A; Tutill, HJ; Purohit, A; Day, JM; Mahon, MF; Reed, MJ; Potter, BV Modification of estrone at the 6, 16, and 17 positions: novel potent inhibitors of 17beta-hydroxysteroid dehydrogenase type 1. J Med Chem 49: 1325-45 (2006)
- Gobec, S; Sova, M; Kristan, K; Rizner, TL Cinnamic acid esters as potent inhibitors of fungal 17beta-hydroxysteroid dehydrogenase--a model enzyme of the short-chain dehydrogenase/reductase superfamily. Bioorg Med Chem Lett 14: 3933-6 (2004)
- Qiu, W; Zhou, M; Mazumdar, M; Azzi, A; Ghanmi, D; Luu-The, V; Labrie, F; Lin, SX Structure-based inhibitor design for an enzyme that binds different steroids: a potent inhibitor for human type 5 17beta-hydroxysteroid dehydrogenase. J Biol Chem 282: 8368-79 (2007)
- Jin, C; Manikumar, G; Kepler, JA; Cook, CE; Allan, GF; Kiddoe, M; Bhattacharjee, S; Linton, O; Lundeen, SG; Sui, Z Synthesis and identification of novel 11beta-aryl-4',5'-dihydrospiro[estra-4,9-diene-17beta,4'-oxazole] analogs with dissociated antiprogesterone activities. Bioorg Med Chem Lett 17: 5754-7 (2007)
- Ashton, MJ; Lawrence, C; Karlsson, JA; Stuttle, KA; Newton, CG; Vacher, BY; Webber, S; Withnall, MJ Anti-inflammatory 17beta-thioalkyl-16alpha,17alpha-ketal and -acetal androstanes: a new class of airway selective steroids for the treatment of asthma. J Med Chem 39: 4888-96 (1997)
- Bydal, P; Luu-The, V; Labrie, F; Poirier, D Steroidal lactones as inhibitors of 17beta-hydroxysteroid dehydrogenase type 5: chemical synthesis, enzyme inhibitory activity, and assessment of estrogenic and androgenic activities. Eur J Med Chem 44: 632-44 (2009)
- Templeton, JF; Ling, Y; Marat, K; LaBella, FS Synthesis and structure-activity relationships of 17beta-substituted 14beta-hydroxysteroid 3-(alpha-L-rhamnopyranoside)s: steroids that bind to the digitalis receptor. J Med Chem 40: 1439-46 (1997)
- Zhao, L; Jin, C; Mao, Z; Gopinathan, MB; Rehder, K; Brinton, RD Design, synthesis, and estrogenic activity of a novel estrogen receptor modulator--a hybrid structure of 17beta-estradiol and vitamin E in hippocampal neurons. J Med Chem 50: 4471-81 (2007)
- Tchédam Ngatcha, B; Luu-The, V; Labrie, F; Poirier, D Androsterone 3alpha-ether-3beta-substituted and androsterone 3beta-substituted derivatives as inhibitors of type 3 17beta-hydroxysteroid dehydrogenase: chemical synthesis and structure-activity relationship. J Med Chem 48: 5257-68 (2005)
- Laplante, Y; Cadot, C; Fournier, MA; Poirier, D Estradiol and estrone C-16 derivatives as inhibitors of type 1 17beta-hydroxysteroid dehydrogenase: blocking of ER+ breast cancer cell proliferation induced by estrone. Bioorg Med Chem 16: 1849-60 (2008)
- Bellavance, E; Luu-The, V; Poirier, D Potent and selective steroidal inhibitors of 17beta-hydroxysteroid dehydrogenase type 7, an enzyme that catalyzes the reduction of the key hormones estrone and dihydrotestosterone. J Med Chem 52: 7488-502 (2009)
- Möller, G; Deluca, D; Gege, C; Rosinus, A; Kowalik, D; Peters, O; Droescher, P; Elger, W; Adamski, J; Hillisch, A Structure-based design, synthesis and in vitro characterization of potent 17beta-hydroxysteroid dehydrogenase type 1 inhibitors based on 2-substitutions of estrone and D-homo-estrone. Bioorg Med Chem Lett 19: 6740-4 (2009)
- Cerri, A; Almirante, N; Barassi, P; Benicchio, A; Fedrizzi, G; Ferrari, P; Micheletti, R; Quadri, L; Ragg, E; Rossi, R; Santagostino, M; Schiavone, A; Serra, F; Zappavigna, MP; Melloni, P 17beta-O-Aminoalkyloximes of 5beta-androstane-3beta,14beta-diol with digitalis-like activity: synthesis, cardiotonic activity, structure-activity relationships, and molecular modeling of the Na(+),K(+)-ATPase receptor. J Med Chem 43: 2332-49 (2000)
- Kortylewicz, ZP; Nearman, J; Baranowska-Kortylewicz, J Radiolabeled 5-iodo-3'-O-(17beta-succinyl-5alpha-androstan-3-one)-2'-deoxyuridine and its 5'-monophosphate for imaging and therapy of androgen receptor-positive cancers: synthesis and biological evaluation. J Med Chem 52: 5124-43 (2010)
- ChEMBL_2281002 Inhibition of rat liver SULT1A1-mediated oestradiol sulfonation incubated for 20 mins
- ChEMBL_340361 (CHEMBL864402) Inhibition of 17beta-HSD3
- ChEMBL_391451 (CHEMBL870480) Inhibition of 17beta-HSD3
- ChEMBL_624973 (CHEMBL1108829) Inhibition of 17beta-HSD3
- ChEMBL_701690 (CHEMBL1656582) Inhibition of 17beta-HSD1
- ChEMBL_768820 (CHEMBL1831802) Inhibition of 17beta-HSD1
- ChEBML_1685728 Inhibition of 17beta-HSD1 (unknown origin)
- ChEMBL_514253 (CHEMBL975500) Inhibition of human placental 17beta-HSD1 assessed as conversion of [3H]estrone to [3H]17beta-estradiol
- ChEMBL_514254 (CHEMBL975501) Inhibition of human placental 17beta-HSD2 assessed as conversion of [3H]17beta-estradiol to [3H]estrone
- ChEMBL_684637 (CHEMBL1285470) Inhibition of human placenta 17beta-HSD2 using [2,4,6,7-3H]-17beta-estradiol as substrate after 10 mins
- ChEMBL_838505 (CHEMBL2078133) TP_TRANSPORTER: inhibition of estradiol-17beta-glucuronide uptake(estradiol-17beta-glucuronide:0.02uM) in OATP1B1-expressing HEK293 cells
- ChEMBL_398762 (CHEMBL908034) Inhibition of human recombinant 17beta-HSD2
- ChEMBL_466329 (CHEMBL929276) Inhibition of human placental 17beta-HSD1
- ChEMBL_466330 (CHEMBL929274) Inhibition of human placental 17beta-HSD2
- ChEMBL_879294 (CHEMBL2208692) Inhibition of human estradiol 17beta-dehydrogenase
- ChEMBL_938953 (CHEMBL2328442) Inhibition of 17beta-HSD2 (unknown origin)
- ChEMBL_988468 (CHEMBL2438117) Inhibition of 17beta-HSD2 (unknown origin)
- ChEMBL_331401 (CHEMBL867043) Displacement of [3H]17beta-estradiol from ERalpha
- ChEMBL_331402 (CHEMBL867044) Displacement of [3H]17beta-estradiol from ERbeta
- ChEMBL_340362 (CHEMBL864403) Inhibition of 17beta-HSD3 by SEAP assay
- ChEMBL_815206 (CHEMBL2025223) Displacement of [3H]-17beta-estradiol from ERalpha
- ChEMBL_815207 (CHEMBL2025224) Displacement of [3H]-17beta-estradiol from ERbeta
- ChEMBL_624972 (CHEMBL1108828) Inhibition of 17beta-HSD3 in human testis microsomes
- ChEMBL_796574 (CHEMBL1937889) Inhibition of 17beta-HSD3 in human testes homogenate
- ChEMBL_796575 (CHEMBL1937890) Inhibition of 17beta-HSD3 in mouse testes homogenate
- ChEMBL_878460 (CHEMBL2187374) Inhibition of human 17beta-HSD1 by HPLC assay
- ChEMBL_2273897 Displacement of [3H]-17beta-estradiol to estrogen receptor (unknown origin)
- ChEMBL_448344 (CHEMBL898603) Displacement of [3H]17beta-estradiol from recombinant human ERalpha
- ChEMBL_448345 (CHEMBL898604) Displacement of [3H]17beta-estradiol from recombinant human ERbeta
- ChEMBL_552851 (CHEMBL958056) Inhibition of human placental 17beta-HSD1 using estrone substrate
- ChEMBL_603517 (CHEMBL1068436) Inhibition of human 17beta-HSD3 expressed in HeLa cells
- ChEMBL_630246 (CHEMBL1106455) Displacement of [3H]estrone from human placental 17beta-HSD1
- ChEMBL_630247 (CHEMBL1106456) Displacement of [3H]estradiol from human placental 17beta-HSD2
- ChEMBL_624968 (CHEMBL1108824) Inhibition of 17beta-HSD3 in Sprague-Dawley rat laydig cells
- ChEMBL_624970 (CHEMBL1108826) Inhibition of 17beta-HSD3 in Sprague-Dawley rat testis microsomes
- ChEMBL_1458227 (CHEMBL3368725) Inhibition of 17beta-HSD2 (unknown origin) expressed in HEK293 cells lysate
- ChEMBL_1458228 (CHEMBL3368726) Inhibition of 17beta-HSD2 (unknown origin) expressed in intact HEK293 cells
- ChEMBL_1458229 (CHEMBL3368727) Inhibition of 17beta-HSD1 (unknown origin) expressed in HEK293 cells lysate
- ChEMBL_1458231 (CHEMBL3368729) Inhibition of 17beta-HSD3 (unknown origin) expressed in intact HEK293 cells
- ChEMBL_552852 (CHEMBL958057) Inhibition of human placental 17beta-HSD2 using 17-beta-estradiol substrate
- ChEMBL_1613002 (CHEMBL3854802) Inhibition of CDK8 in human 7dF3 cells preincubated for 2 hrs followed by beta-oestradiol addition measured after 24 hrs by luciferase reporter gene assay
- ChEBML_1581929 Displacement of [3H]-17beta-estradiol from human recombinant ERalpha expressed in rabbit reticulocytes
- ChEBML_1581930 Displacement of [3H]-17beta-estradiol from human recombinant ERbeta expressed in rabbit reticulocytes
- ChEMBL_1484215 (CHEMBL3537876) Inhibition of SULT1A1 in human MCF7 cells assessed as 17beta-estradiol sulfation
- ChEMBL_325410 (CHEMBL858655) Inhibitory activity against type 1 17beta-HSD expressed in transfected HEK293 cells
- ChEMBL_618687 (CHEMBL1102465) Displacement of [3H]17beta-estradiol from human ERalpha expressed in SF9 cells
- ChEMBL_2280986 Displacement of [3H]-17beta-estradiol from bovine uterine estrogen receptor by competitive binding assay
- ChEMBL_326622 (CHEMBL863381) Displacement of [3H]17beta-estradiol from recombinant human ERalpha expressed in 293T cells
- ChEMBL_326623 (CHEMBL868723) Displacement of [3H]17beta-estradiol from recombinant human ERbeta expressed in 293T cells
- ChEMBL_475833 (CHEMBL940137) Inhibition of 17beta-HSD1 expressed in intact human T47D cells after 30 mins
- ChEMBL_700705 (CHEMBL1645744) Inhibition of [3H]E2 binding to human placental 17beta-HSD2 after 20 mins
- ChEMBL_700706 (CHEMBL1645745) Inhibition of [3H]E1 binding to human placental 17beta-HSD1 after 20 mins
- ChEMBL_1589660 (CHEMBL3829689) Binding affinity to CDK8 (unknown origin) expressed in human 7dF3 cells preincubated for 2 hrs followed by beta-oestradiol addition measured after 24 hrs by luciferase reporter gene assay
- ChEMBL_1487741 (CHEMBL3533735) Inhibition of OATP1B1 (unknown origin) expressed in HEK293 cells using estradiol-17beta-glucuronide substrate
- ChEMBL_618686 (CHEMBL1102464) Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting
- ChEMBL_1436369 (CHEMBL3389572) Inhibition of OATP1B1 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrate
- ChEMBL_1436370 (CHEMBL3389573) Inhibition of OATP1B3 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrate
- ChEMBL_544137 (CHEMBL1014266) Inhibition of 17beta-HSD1 assessed as conversion of [14C]estradiol to [14C]estrone using NADP+
- ChEMBL_684635 (CHEMBL1285468) Inhibition of human placenta 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins
- ChEMBL_787297 (CHEMBL1919075) Inhibition of human placental microsomal 17beta-HSD2 using [3H]E2 as substrate by HPLC analysis
- ChEMBL_787298 (CHEMBL1919076) Inhibition of human placental cytosolic 17beta-HSD1 using [3H]E1 as substrate by HPLC analysis
- ChEMBL_877515 (CHEMBL2184039) Inhibition of 17beta-HSD1 assessed as conversion of estrone to estradiol by scintillation counting method
- ChEMBL_2193599 (CHEMBL5105959) Displacement of fluorescent labeled 17beta-estradiol from human ERbeta ligand binding domain by TR-FRET assay
- ChEMBL_715249 (CHEMBL1664450) Displacement of [3H]E2 from human placental 17beta-HSD2 after 10 mins by fluid scintillation counting
- ChEMBL_715250 (CHEMBL1664451) Displacement of [3H]E1 from human placental 17beta-HSD1 after 10 mins by fluid scintillation counting
- ChEMBL_752205 (CHEMBL1786581) Inhibition of fluorescence-labeled 17beta-estradiol binding to ERalpha receptor after 2 hrs by fluorometric analysis
- ChEMBL_787302 (CHEMBL1919080) Inhibition of 17beta-HSD2 in human MDA-MB-231 cells after 3.5 hrs by HPLC analysis
- ChEMBL_1439099 (CHEMBL3384914) Displacement of [3H]E2 from human placental microsomal 17beta-HSD2 after 20 mins by cell-free assay
- ChEMBL_1717009 (CHEMBL4132009) Antagonist activity at ER in human MCF7 cells assessed as inhibition of 17beta-estradiol-induced cell proliferation
- ChEMBL_2074723 (CHEMBL4730257) Displacement of [3H]-17beta-E2 from human ERbeta LBD by solid phase competitive radio ligand binding assay
- ChEMBL_684640 (CHEMBL1285473) Inhibition of 17beta-HSD1 in human T47D cells using [2,4,6,7-3H]-estrone as substrate after 30 mins
- ChEMBL_544140 (CHEMBL1013421) Inhibition of 17beta-HSD1 expressed in HEK 293 cells assessed as conversion of [14C]estrone to [14C]estradiol
- ChEMBL_634656 (CHEMBL1118096) Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay
- ChEMBL_634657 (CHEMBL1118530) Displacement of [3H]-cortisone human 17beta-HSD1 expressed in HEK293 cells after 30 mins by scintillation proximity assay
- ChEMBL_715308 (CHEMBL1664595) Displacement of [3H]E1 from 17beta-HSD1 in human T47D cells after 10 mins by fluid scintillation counting
- ChEMBL_1490069 (CHEMBL3536010) Inhibition of OATP1B3-mediated [3H]estradiol 17beta-glucuronide uptake in human OATP1B3 expressing HEK293/PDZK1 cells by scintillation counting
- ChEMBL_1510493 (CHEMBL3607519) Inhibition of 17beta-HSD3 in rat testes microsomes using [14C]-4-androstene-3,17-dione as substrate after 2 hrs
- ChEMBL_1717005 (CHEMBL4132005) Displacement of [3H]17beta-estradiol from ER in human MCF7 cells after 18 hrs by microbeta scintillation counting method
- ChEMBL_468301 (CHEMBL930592) Inhibition of 17beta-HSD1 in human T47D cells assessed as inhibition of transformation of [14C]-estrone into [14C]estrogen
- ChEMBL_555024 (CHEMBL957999) Inhibition of human recombinant 17beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol
- ChEMBL_555026 (CHEMBL958789) Inhibition of human recombinant 17beta-HSD2 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol
- ChEMBL_796573 (CHEMBL1937888) Inhibition of human 17beta-HSD3 expressed in HeLa cells assessed as conversion of 4-androstene-3,17-dione to testosterone
- ChEMBL_1491711 (CHEMBL3536903) Inhibition of human MRP2-mediated estradiol-17beta-D-glucuronide formation using inside-out membrane vesicles by LC-MS/MS analysis
- ChEMBL_1619744 (CHEMBL3861913) Inhibition of purified human placental cytosolic 17beta-HSD1 using [3H]-E1 substrate and NADH by HPLC based radioactive displacement assay
- ChEMBL_1619745 (CHEMBL3861914) Inhibition of purified human placental microsomal 17beta-HSD2 using [3H]-E2 substrate and NAD+ by HPLC based radioactive displacement assay
- ChEMBL_1861100 (CHEMBL4361956) Inhibition of mouse liver homogenate 17beta-HSD2 using [3H]-E2 as substrate in presence of NAD+ by radio-HPLC analysis
- ChEMBL_1861111 (CHEMBL4361967) Inhibition of rat liver homogenate 17beta-HSD2 using [3H]-E2 as substrate in presence of NAD+ by radio-HPLC analysis
- ChEMBL_1863754 (CHEMBL4364729) Inhibition of mouse liver homogenate 17beta-HSD2 using [3H]-E2 as substrate in presence of NAD+ by radio-HPLC analysis
- ChEMBL_1898529 (CHEMBL4400644) Inhibition of mouse liver homogenate 17beta-HSD2 using [3H]-E2 as substrate in presence of NAD+ by radio-HPLC analysis
- ChEMBL_2217337 (CHEMBL5130469) Inhibition of 17beta-HSD1 in human T47D cells using [3H]E1S as substrate measured after 24 hrs by HPLC analysis
- ChEMBL_2493908 Inhibition of human recombinant 17beta-HSD10 expressed in bacterial expression system assessed as decrease in NADH using acetoacetyl-CoA as substrate
- ChEMBL_544139 (CHEMBL1013420) Inhibition of 17beta-HSD1 expressed in HEK 293 cells assessed as conversion of [14C]estrone to [14C]estradiol using NADH
- ChEMBL_544141 (CHEMBL1013422) Inhibition of 17beta-HSD1 expressed in HEK 293 cells assessed as conversion of [14C]estrone to [14C]estradiol using NADPH
- ChEMBL_975807 (CHEMBL2415373) Inhibition of human 17beta-HSD5 expressed in HEK293 cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs
- ChEMBL_1363487 (CHEMBL3291709) Inhibition of human placental 17beta-HSD2 microsomal fraction using tritiated estradiol as substrate assessed as formation of estrone by HPLC analysis
- ChEMBL_1363489 (CHEMBL3291711) Inhibition of human placental 17beta-HSD1 cytosolic fraction using tritiated estrone as substrate assessed as formation of estradiol by HPLC analysis
- ChEMBL_1363494 (CHEMBL3291985) Inhibition of mouse liver 17beta-HSD2 microsomal fraction using tritiated estradiol as substrate assessed as formation of estrone by HPLC analysis
- ChEMBL_1484074 (CHEMBL3537109) Inhibition of human OATP1B1 expressed in CHO cells using [3H]estradiol 17beta-D-glucuronide as substrate by liquid scintillation counting analysis
- ChEMBL_1484212 (CHEMBL3537873) Inhibition of human SULT1A1 expressed in Escherichia coli assessed as 17beta-estradiol sulfation at 100 nM by Michaelis-Menten equation analysis
- ChEMBL_1541061 (CHEMBL3744292) Inhibition of 17beta-HSD2 in human placental microsomal fraction using E2/NAD+ as substrate/cofactor after 20 mins by HPLC analysis
- ChEMBL_1541062 (CHEMBL3744293) Inhibition of 17beta-HSD1 in human placental cytosolic fraction using E1/NADH as substrate/cofactor after 10 mins by HPLC analysis
- ChEMBL_1861097 (CHEMBL4361953) Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate in presence of NAD+ by radio-HPLC analysis
- ChEMBL_1861098 (CHEMBL4361954) Inhibition of human placental cytosolic fraction 17beta-HSD1 using [3H]-E1 as substrate in presence of NAD+ by radio-HPLC analysis
- ChEMBL_1863749 (CHEMBL4364724) Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate in presence of NAD+ by radio-HPLC analysis
- ChEMBL_1863752 (CHEMBL4364727) Inhibition of human placental cytosolic fraction 17beta-HSD1 using [3H]-E1 as substrate in presence of NAD+ by radio-HPLC analysis
- ChEMBL_1898526 (CHEMBL4400641) Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate in presence of NAD+ by radio-HPLC analysis
- ChEMBL_1898527 (CHEMBL4400642) Inhibition of human placental cytosolic fraction 17beta-HSD1 using [3H]-E1 as substrate in presence of NAD+ by radio-HPLC analysis
- ChEMBL_527767 (CHEMBL978703) Agonist activity at human ERalpha expressed in african green monkey CV1 cells by luciferase reporter gene assay relative to 17beta estradiol
- ChEMBL_938945 (CHEMBL2328434) Inhibition of 17beta-HSD2 in human MDA-MB-231 cells using [3H]E2 as substrate after 6 hrs by HPLC analysis
- ChEMBL_975806 (CHEMBL2415372) Inhibition of human 17beta-HSD5 expressed in human CWR22R cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs
- ChEMBL_1861108 (CHEMBL4361964) Inhibition of 17beta-HSD2 in human MDA-MB-231 cells using [3H]-E2 as substrate after 6 hrs by radio-HPLC analysis
- ChEMBL_794210 (CHEMBL1932035) Inhibition of human microsomal 17beta-HSD2 in cell-free system using [2,4,6,7-3H]E2 as substrate after 20 mins by radioflow detector
- ChEMBL_794211 (CHEMBL1932036) Inhibition of human cytosolic 17beta-HSD1 in cell-free system using [2,4,6,7-3H]E1 as substrate after 10 mins by radioflow detector
- ChEMBL_938947 (CHEMBL2328436) Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis
- ChEMBL_938948 (CHEMBL2328437) Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis
- ChEMBL_1367282 (CHEMBL3300203) Inhibition of human placental 17beta-HSD1 cytosolic fraction using [2, 4, 6, 7-3H]-estrone as substrate after 10 mins by HPLC analysis
- ChEMBL_1367283 (CHEMBL3300204) Inhibition of human placental 17beta-HSD2 microsomal fraction using [2, 4, 6, 7-3H]-estradiol as substrate after 20 mins by HPLC analysis
- ChEMBL_1524155 (CHEMBL3631240) Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis
- ChEMBL_1524156 (CHEMBL3631241) Inhibition of human placental microsomal 17beta HSD2 using unlabeled- and labelled [2,4,6,7-3H]-E2 as substrate incubated for 20 mins by HPLC analysis
- ChEMBL_1524162 (CHEMBL3631247) Inhibition of mouse liver microsomal 17beta HSD2 using unlabeled- and labelled [2,4,6,7-3H]-E2 as substrate incubated for 20 mins by HPLC analysis
- ChEMBL_1524167 (CHEMBL3631252) Inhibition of rat liver microsomal 17beta HSD2 using unlabeled- and labelled [2,4,6,7-3H]-E2 as substrate incubated for 20 mins by HPLC analysis
- ChEMBL_1700821 (CHEMBL4051803) Inhibition of human placental cytosolic 17beta-HSD1 using [3H]-E1/E1 substrate and NADH after 10 mins by HPLC based radio-detection method
- ChEMBL_1700822 (CHEMBL4051804) Inhibition of human placental cytosolic 17beta-HSD2 using [3H]-E2/E2 substrate and NAD+ after 20 mins by HPLC based radio-detection method
- ChEMBL_1861112 (CHEMBL4361968) Inhibition of recombinant rat 17beta-HSD1 expressed in HEK293 cells using [3H]-E1 as substrate in presence of NAD+ by radio-HPLC analysis
- ChEMBL_1898532 (CHEMBL4400647) Inhibition of recombinant mouse 17beta-HSD1 expressed in HEK293 cells using [3H]-E1 as substrate in presence of NAD+ by radio-HPLC analysis
- ChEMBL_1898536 (CHEMBL4400651) Inhibition of 17beta-HSD2 in human using MDA-MB-231 cells using [3H]-E2 as substrate after 6 hrs by cell-based assay
- ChEMBL_2324327 Inhibition of human placental cytosolic fraction 17beta-HSD1 using [3H]E1 as substrate measured after 10 mins in presence of NADH by HPLC analysis
- ChEMBL_2324328 Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]E2 as substrate measured after 20 mins in presence of NAD+ by HPLC analysis
- ChEMBL_619853 (CHEMBL1106730) Inhibition of human 17beta-HSD7 expressed in HEK293 cells assessed as inhibition of reduction of [14C]estrone into [14C]estradiol after 7 hrs
- ChEMBL_701686 (CHEMBL1656578) Displacement of [3H]estrone human recombinant 17beta-HSD1 expressed in Escherichia coli BL21 (DE3)-RIL by scintillation counting in presence of bacterial homogenate
- ChEMBL_768581 (CHEMBL1832600) Inhibition of 17beta-HSD1 in human T47D cells expressing estrogen receptor assessed as conversion of [14C]-E1 to [14C]-E2 after 24 hrs
- ChEMBL_1438124 (CHEMBL3388499) Inhibition of AKR1C3-mediated androsterone metabolism in human A549 cells assessed as 5alpha-androstane-3alpha, 17beta-diol after 24 hrs by LC/MS analysis
- ChEMBL_1494637 (CHEMBL3529887) Inhibition of OATP1B1 (unknown origin) expressed in HEK293 cells assessed as reduction of [3H]estradiol-17beta-glucuronide uptake after 3 mins by beta-counting
- ChEMBL_1494638 (CHEMBL3529888) Inhibition of OATP1B3 (unknown origin) expressed in HEK293 cells assessed as reduction of [3H]estradiol-17beta-glucuronide uptake after 3 mins by beta-counting
- ChEMBL_1646099 (CHEMBL3995155) Inhibition of human placental microsomal 17beta-HSD2 using [2,4,6,7-3H]-E2 as substrate after 20 mins in presence of NAD+ by RP-HPLC method
- ChEMBL_1744042 (CHEMBL4178552) Inhibition of human 17beta-HSD2 expressed in HEK293 cell lysates incubated for 10 mins using [2,4,6,7-3H]-estradiol and NAD+ by scintillation counting method
- ChEMBL_1744044 (CHEMBL4178554) Inhibition of human 17beta-HSD1 expressed in HEK293 cell lysates incubated for 10 mins using [2,4,6,7-3H]-estrone and NADPH by scintillation counting method
- ChEMBL_2173334 (CHEMBL5058468) Inhibition of human placental cytosolic fraction 17beta-HSD1 using [3H]-E1 as substrate in presence of NADH measured after 10 mins by HPLC method
- ChEMBL_2173335 (CHEMBL5058469) Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate in presence of NAD+ measured after 20 mins by HPLC method
- ChEMBL_2217338 (CHEMBL5130470) Inhibition of human placental cytosolic fraction 17beta-HSD1 using [3H]-E1 as substrate in presence of NADH measured after 10 mins by HPLC method
- ChEMBL_2217339 (CHEMBL5130471) Inhibition of human placental microsomal fraction 17beta-HS2 using [3H]-E2 as substrate in presence of NAD+ measured after 10 mins by HPLC method
- ChEMBL_2493915 Inhibition of recombinant 17beta-HSD10 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as residual activity using acetoacetyl-CoA as substrate by SAAC method
- ChEMBL_701687 (CHEMBL1656579) Displacement of [3H]estrone human recombinant 17beta-HSD1 expressed in Escherichia coli BL21 (DE3)-RIL by competitive inhibition assay in presence of bacterial homogenate
- ChEMBL_1337645 (CHEMBL3242833) Antagonist activity at human Gal4-fused ER-alpha expressed in HEK293 cells assessed as inhibition of 17beta-estradiol-induced effect by luciferase reporter gene assay
- ChEMBL_1337649 (CHEMBL3242837) Antagonist activity at human Gal4-fused ER-beta expressed in HEK293 cells assessed as inhibition of 17beta-estradiol-induced effect by luciferase reporter gene assay
- ChEMBL_1789613 (CHEMBL4261347) Inhibition of human placental cytosolic 17beta-HSD1 using [3H]-E1 as substrate after 10 mins in presence of NADPH by radio-flow detector based analysis
- ChEMBL_1789617 (CHEMBL4261351) Inhibition of mouse microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector based analysis
- ChEMBL_988473 (CHEMBL2438122) Inhibition of human placenta 17beta-HSD1 cytosolic fraction using unlabeled, [2,4,6,7-3H]-estrone as substrate after 20 mins by HPLC analysis in presence of NADH
- ChEMBL_988475 (CHEMBL2438124) Inhibition of human placenta 17beta-HSD2 microsomal fraction using unlabeled, [2,4,6,7-3H]-estradiol as substrate after 20 mins by HPLC analysis in presence of NAD+
- ChEBML_1685205 Inhibition of recombinant human 17beta-HSD2 expressed in HEK293 cells using estradiol as substrate after 10 mins in presence of radiolabeled tracer substrate by scintillation counting method
- ChEMBL_1484075 (CHEMBL3537110) Inhibition of human OATP1B3 expressed in BacMam baculovirus virus infected HEK MSR2 cells using [3H]estradiol 17beta-D-glucuronide as substrate by liquid scintillation counting analysis
- ChEMBL_1524165 (CHEMBL3631250) Inhibition of recombinant mouse 17beta HSD1 expressed in HEK293 cells using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis
- ChEMBL_1524166 (CHEMBL3631251) Inhibition of recombinant rat 17beta HSD1 expressed in HEK293 cells using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis
- ChEMBL_1789612 (CHEMBL4261346) Inhibition of human placental microsomal fraction 17beta-HSD2 using [3H]-E2 as substrate after 20 mins in presence of NAD+ by radio-flow detector based analysis
- ChEMBL_861317 (CHEMBL2173705) Inhibition of human placenta 17beta-HSD1 cytosolic fraction using unlabelled and [2,4,6,7-3H]estrone as substrate after 10 mins by HPLC analysis in presence of NADH
- ChEMBL_861318 (CHEMBL2173706) Inhibition of human placenta 17beta-HSD2 microsomal fraction using unlabelled and [2,4,6,7-3H]estradiol as substrate after 20 mins by HPLC analysis in presence of NAD+
- ChEMBL_879539 (CHEMBL2208948) Inhibition of 17beta-HSD1 in human T47D cells assessed as decrease in transformation of [14C]estrone to [14C]-estradiol after 24 hrs by thin layer chromatography
- Inhibition of 17beta-HSD1 Tritiated E1 was incubated with 17beta-HSD1, cofactor, and inhibitor. The amount of labeled E2 formed was quantified by HPLC. Detection and quantification of the steroids were performed using a radioflow detector (Berthold Technologies, Bad Wildbad). The conversion rate was calculated: %conversion = (%E2/ (%E2 +%E1) x 100). Each value was calculated from at least three independent experiments.
- Inhibition of 17beta-HSD2 Tritiated E2 was incubated with 17beta-HSD2, cofactor, and inhibitor. The amount of labeled E1 formed was quantified by HPLC. Detection and quantification of the steroids were performed using a radioflow detector (Berthold Technologies, Bad Wildbad). The conversion rate was calculated: %conversion = (%E1/ (%E2 +%E1) x 100). Each value was calculated from at least three independent experiments.
- ChEMBL_565719 (CHEMBL962458) Inhibition of human 17beta-HSD5 expressed in HEK293 cells assessed as enzyme-mediated transformation of [14C]-4-androstene-3,17-dione in to [14C]-testosterone after 18 hrs
- ChEMBL_1274566 (CHEMBL3091166) Inhibition of 17beta-HSD3 in rat testes microsomal fraction using [14C]-4-androstene-3,17-dione as substrate assessed as testosterone formation after 2 hrs by thin layer chromatography
- ChEMBL_1904874 (CHEMBL4407232) Inhibition of 17beta-HSD3 (unknown origin) expressed in human LNCAP cells assessed as reduction in [14C]-testosterone formation from [14C]-4-androstene-3,17-dione measured after 1 hr
- ChEMBL_1904878 (CHEMBL4407236) Inhibition of 17beta-HSD3 in Sprague-Dawley rat testes microsomal fraction assessed as reduction in [14C]-testosterone formation from [14C]-4-androstene-3,17-dione measured after 2 hrs
- ChEMBL_786680 (CHEMBL1921533) Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presence of NADH as cofactor
- ChEMBL_1339080 (CHEMBL3243177) Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional activation after 24 hrs by ERE-luciferase reporter gene assay
- ChEMBL_762463 (CHEMBL1816733) Inhibition of 17Beta-HSD3 expressed in intact HEK293 cells assessed as transformation of [14C]-4-androstene-3,17-dione into [14C]-testosterone in presence of NADPH after 1 hr incubation
- ChEMBL_1577036 (CHEMBL3807012) Antagonist activity at ERalpha (unknown origin) transfected in 17beta-estradiol induced-HEK293 cells assessed as inhibition of estradiol-mediated protein transcriptional activity after 8 hrs by Luciferase reporter gene assay
- ChEMBL_1646098 (CHEMBL3995154) Inhibition of human placental cytosolic 17beta-HSD1 assessed as reduction in activation of [2,4,6,7-3H]-E1 substrate to E2 after 10 mins in presence of NADH by RP-HPLC method
- ChEMBL_2381114 Inhibition of recombinant 17beta-HSD10 (unknown origin) using E2 as substrate and NAD+ as cofactor preincubated for 5 mins followed by substrate addition and measured after 20 mins by fluorometric assay
- ChEMBL_2381115 Inhibition of recombinant 17beta-HSD10 (unknown origin) using ALLOP as substrate and NAD+ as cofactor preincubated for 5 mins followed by substrate addition and measured after 20 mins by fluorometric assay
- ChEMBL_861311 (CHEMBL2173699) Inhibition of 17beta-HSD1 in human T47D cells using unlabelled and [2,4,6,7-3H]estrone as substrate preincubated for 30 mins prior to substrate addition measured 0.5 hrs post substrate addition
- ChEMBL_1670787 (CHEMBL4020675) Binding affinity to recombinant human GFP-fused ERalpha LBD (301 to 553 residues) expressed in Escherichia coli BL21(DE3) after 40 mins in presence of 17beta-estradiol by fluorescence polarization assay
- ChEMBL_1670788 (CHEMBL4020676) Binding affinity to recombinant human GFP-fused ERbeta LBD (259 to 498 residues) expressed in Escherichia coli BL21(DE3) after 40 mins in presence of 17beta-estradiol by fluorescence polarization assay
- ChEMBL_1700827 (CHEMBL4051809) Inhibition of 17beta-HSD1 in human T47D cells preincubated for 1 hr followed by addition of [3H]-E1/E1 as substrate measured after 30 mins by HPLC based radio-detection method
- ChEMBL_1739715 (CHEMBL4155465) Binding affinity to recombinant 17beta-HSD14 (unknown origin) expressed in Escherichia coli BL21 pLysS strain using E2 as substrate in presence of NAD measured continuously for 15 mins by fluorimetric method
- ChEMBL_2381116 Mixed type inhibition of recombinant 17beta-HSD10 (unknown origin) using E2 as substrate and NAD+ as cofactor preincubated for 5 mins followed by substrate addition and measured after 20 mins by fluorometric assay
- ChEMBL_1859158 (CHEMBL4360014) Antagonist activity at human full-length ERalpha expressed in non-human mammalian expression system assessed as inhibition of 17beta-estradiol-induced response measured after 22 to 24 hrs by luciferase reporter gene assay
- ChEMBL_1859159 (CHEMBL4360015) Antagonist activity at human full-length ERbeta expressed in non-human mammalian expression system assessed as inhibition of 17beta-estradiol-induced response measured after 22 to 24 hrs by luciferase reporter gene assay
- ChEMBL_2324321 Inhibition of human 17beta-HSD1 expressed in human T47D cells using [3H]E1 as substrate incubated for 1 hr followed by substrate addition measured after 40 mins in presence of E1 by HPLC analysis
- ChEMBL_1668112 (CHEMBL4018000) Antagonist activity at estrogen receptor in mouse GT1-7 cells harboring beta-galactosidase reporter gene assessed as reduction in 17beta-estradiol induced response measured after 20 to 24 hrs by luciferase reporter gene assay
- ChEMBL_1678645 (CHEMBL4028922) Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and GSH measured after 20 mins by membrane vesicle transport assay
- ChEMBL_1678647 (CHEMBL4028924) Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and GSH measured after 20 mins by membrane vesicle transport assay
- ChEMBL_1484073 (CHEMBL3537108) Inhibition of human MRP2 expressed in baculovirus-infected Sf9 cell membrane vesicle using [3H]estradiol 17beta-D-glucuronide as substrate preincubated with vesicles for 5 mins prior to substrate addition by liquid scintillation counting analysis
- ChEMBL_1678646 (CHEMBL4028923) Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and GSH measured after 10 mins by membrane vesicle transport assay
- ChEMBL_786678 (CHEMBL1921531) Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by radio flow detector-based HPLC analysis in presence of NAD+ as cofactor
- ChEMBL_1792852 (CHEMBL4264771) Inhibition of recombinant mouse full-length 17beta-HSD10 (2 to 261 residues) assessed as reduction in transformation of [14C]-E2 to E1 after 48 hrs in presence of 1 mM NAD+ cofactor by liquid scintillation counting method
- ChEMBL_2113959 (CHEMBL4822809) Antagonist activity at ERalpha (unknown origin) expressed in human HEK293T cells assessed as inhibition of 17beta-estradiol-induced transcriptional activities by measuring ERE driven reporter gene expression measured after 24 hrs by dual luciferase reporter gene assay
- ChEMBL_2113960 (CHEMBL4822810) Antagonist activity at ERbeta (unknown origin) expressed in human HEK293T cells assessed as inhibition of 17beta-estradiol-induced transcriptional activities by measuring ERE driven reporter gene expression measured after 24 hrs by dual luciferase reporter gene assay
- ChEMBL_1792848 (CHEMBL4264767) Inhibition of recombinant mouse full-length 17beta-HSD10 (2 to 261 residues) assessed as reduction in transformation of [3H]-ALLOP to 5alpha-DHP after 48 hrs in presence of 1 mM NAD+ cofactor by liquid scintillation counting method
- ChEMBL_2381121 Inhibition of 17beta-HSD10 (unknown origin) expressed in HEK293 cells using (-)-CHANA probe as substrate assessed as decrease in fluorescence intensity of CHANK production preincubated for 20 hrs followed by probe addition and measured after 2 hrs by fluorescence based analysis
- Binding Assay The competition assay was performed in a 96-well plate (polystyrene*) which binds <2.0% of the total input [3H]-17beta-estradiol and each data point was gathered in triplicate. 100 uG/100 uL of the receptor preparation was aliquoted per well. A saturating dose of 2.5 nM [3H]17beta-estradiol+competitor (or buffer) in a 50 uL volume was added in the preliminary competition when 100x and 500x competitor were evaluated, only 0.8 nM [3H]17beta-estradiol was used. The plate was incubated at room temperature for 2.5 h. At the end of this incubation period 150 uL of ice-cold dextran coated charcoal (5% activated charcoal coated with 0.05% 69K dextran) was added to each well and the plate was immediately centrifuged at 99 g for 5 minutes at 4 C. 200 uL of the supernatant solution was then removed for scintillation counting. Samples were counted to 2% or 10 minutes, whichever occurs first.
- ER-alpha Radioligand Binding Assay and ERE-Luciferase Reporter Assay. Radioligand binding assay was performed by using 96-well microtiterplates containing ER, 17beta-estradiol, and the test compound to be tested and SPAbeads. After incubation at room temperature for 2-4 h, the reaction was terminated by centrifugation. The radioactivity was counted in a Packard Topcount scintillation counter. Nonspecific binding was defined as the remaining radioactivity in the presence of 10 uM nonradioactive 17beta-estradiol. Assays were performed in triplicate. HELNalpha, a human cervix adenocarcinoma cell line derived from HeLa cells stably transfected with the reporter gene and the expression plasmid ERalpha, were used to quantify the antiestrogenic and estrogenic effects of compounds on ERE
- ER-alpha Radioligand Binding Assay and ERE-Luciferase Reporter Assay. Radioligand binding assay was performed by using 96-well microtiterplates containing ER, 17beta-estradiol, and the test compound to be tested and SPAbeads. After incubation at room temperature for 2-4 h, the reaction was terminated by centrifugation. The radioactivity was counted in a Packard Topcount scintillation counter. Nonspecific binding was defined as the remaining radioactivity in the presence of 10 uM nonradioactive 17beta-estradiol. Assays were performed in triplicate. HELNalpha, a human cervix adenocarcinoma cell line derived from HeLa cells stably transfected with the reporter gene and the expression plasmid ERalpha, were used to quantify the antiestrogenic and estrogenic effects of compounds on ERE.
- ER-beta Radioligand Binding Assay and ERE-Luciferase Reporter Assay. Radioligand binding assay was performed by using 96-well microtiterplates containing ER, 17beta-estradiol, and the test compound to be tested and SPAbeads. After incubation at room temperature for 2-4 h, the reaction was terminated by centrifugation. The radioactivity was counted in a Packard Topcount scintillation counter. Nonspecific binding was defined as the remaining radioactivity in the presence of 10 uM nonradioactive 17beta-estradiol. Assays were performed in triplicate. HELNbeta, a human cervix adenocarcinoma cell line derived from HeLa cells stably transfected with the reporter gene and the expression plasmid ERbeta, were used to quantify the antiestrogenic and estrogenic effects of compounds on ERE.
- Estrogen Receptor Binding Assay and Ishikawa Assay The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determined by saturation binding to ER receptors. The IC50 values for compounds were converted to Ki using Cheng-Prusoff equation. Estrogenic stimulation and antagonism were measured in Ishikawa human endometrial tumor cells by alkaline phosphatase quantitation. The data were fitted to a linear interpolation to derive IC50 values for the antagonist mode and a percentage efficacy was calculated that blocks the 17beta-estradiol (1nM) stimulus.
- Enzymatic Assay (Inhibition of Type 1 17beta-HSD) The enzymatic reaction was performed in the reaction buffer containing substrate, [14C]-estrone, and the test inhibitors. After the reaction, radiolabeled steroids were extracted from the reaction mixture, and solvent was evaporated to dryness. Steroids were dissolved and separated on TLC plates. Radioactivity signals were detected and quantified using a PhosphoImager (Sunny Vale, CA). The percentage of transformation of [14C]-E1 into [14C]-E2 was calculated. The IC50 values were calculated using an unweighted iterative least-squares method for four-parameter logistic curve fitting (DE50 program, CHUL Research Center, Quebec).
- Enzyme Inhibition on the Conversion of E2 to E1 by 17beta-HSD1 For steady-state kinetic study of hybrid inhibitors, a Fluorolog 3 instrument was used to monitor the fluorescent signal of NADPH formed during estradiol oxidation, through which the substrate conversion is monitored. The excitation wavelength was 340 nm, and the emission wavelength was 460 nm. A standard curve monitoring the increase of fluorescence (cps) caused by an increase of NADPH revealed that the formation of 1 uM NADPH corresponds to a 316,000 cps increase. The Km for E2 in the absence of EM-1745, and four apparent Km values in the presence of 0.5, 2, 4, and 7 nM EM-1745 were obtained. Ki was further determined by a plot of Km vs. the inhibitor concentration.
- 17beta-HSD13 Biochemical Assay More specifically, recombinant 17β-HSD13 protein was assayed in a buffer containing 200 mM Tris pH 7.5, 0.01% Triton X-100, and 0.02% BSA into a 384-well assay plate. Compounds were incubated with 17β-HSD13 (final 50 nM) and NAD+ (final 10 mM) at room temperature for 1 h prior to substrate addition. The assay reaction was then initiated by addition of β-estradiol (final 20 μM), and the reaction mixture was incubated for 2 hours at room temperature. Product formation was detected with chemiluminescence by adding equal volume of NAD+/NADH Glo reagent (Promega, #G9062) and read on a PHERAstar microplate reader (BMG LABTECH). IC50 values were determined with GraphPad Prism®, where log-transformed concentration values and the inhibition data were fitted to a four-parameter logistic equation. Y=Bottom+(Top−Bottom)/(1+10{circumflex over ( )}((Log IC50−X)*HillSlope)).
 hombreol 3alpha,17beta-dihydroxy-5alpha-androstane 5alpha-androstane-3alpha,17beta-diol (3alpha,5alpha,17beta)-androstane-3,17-diol CHEMBL335062 BDBM50093445
hombreol 3alpha,17beta-dihydroxy-5alpha-androstane 5alpha-androstane-3alpha,17beta-diol (3alpha,5alpha,17beta)-androstane-3,17-diol CHEMBL335062 BDBM50093445 CHEMBL260660 BDBM50423533 Dihydroequilenin-17Beta
CHEMBL260660 BDBM50423533 Dihydroequilenin-17Beta 3beta,17beta-Dihydroxyandrost-5-ene CHEMBL440283 Androstenediol (3beta,17beta)-androst-5-ene-3,17-diol 3beta,17beta-Dihydroxy-5-androstene hermaphrodiol Androst-5-ene-3beta,17beta-diol androst-5-enediol BDBM50223237
3beta,17beta-Dihydroxyandrost-5-ene CHEMBL440283 Androstenediol (3beta,17beta)-androst-5-ene-3,17-diol 3beta,17beta-Dihydroxy-5-androstene hermaphrodiol Androst-5-ene-3beta,17beta-diol androst-5-enediol BDBM50223237 17beta-hydroxy-4-estren-3-one 17beta-hydroxyestr-4-en-3-one CHEMBL757 Nandrolone BDBM50080092 (17beta)-17-hydroxyestr-4-en-3-one 19-Nortestosterone 19-Norandrostenolone 4-estren-17beta-ol-3-one 17beta-hydroxy-19-nor-4-androsten-3-one
17beta-hydroxy-4-estren-3-one 17beta-hydroxyestr-4-en-3-one CHEMBL757 Nandrolone BDBM50080092 (17beta)-17-hydroxyestr-4-en-3-one 19-Nortestosterone 19-Norandrostenolone 4-estren-17beta-ol-3-one 17beta-hydroxy-19-nor-4-androsten-3-one 17Beta-HSD Inhibitor, 3 BDBM85468
17Beta-HSD Inhibitor, 3 BDBM85468 BDBM50423526 CHEMBL121458 17Beta-Dihydroequillin 7-Dehydro-Estradiol
BDBM50423526 CHEMBL121458 17Beta-Dihydroequillin 7-Dehydro-Estradiol 3ALPHA,17BETA-DIHYDROXY-5BETA-ANDROSTAN-4-ONE BDBM50441293
3ALPHA,17BETA-DIHYDROXY-5BETA-ANDROSTAN-4-ONE BDBM50441293 BDBM91715 17beta-Hydroxy-5alpha-androstan-3,6-dione, 4
BDBM91715 17beta-Hydroxy-5alpha-androstan-3,6-dione, 4 19-Norethisterone Primolut-N BDBM50148732 17alpha-ethinylestra-4-en-17beta-ol-3-one 19-Nor-17alpha-ethynyl-17beta-hydroxy-4-androsten-3-one Norethisteron NORETHINDRONE norethisterone 17alpha-ethynyl-19-nor-4-androsten-17beta-ol-3-one 19-nor-17alpha-ethynyltestosterone 4-estren-17alpha-ethynyl-17beta-ol-3-one 17alpha-ethinyl-19-nortestosterone 17-ethynyl-17beta-hydroxyestr-4-en-3-one CHEMBL1162 17beta-hydroxy-19-norpregn-4-en-20-yn-3-one Micronor
19-Norethisterone Primolut-N BDBM50148732 17alpha-ethinylestra-4-en-17beta-ol-3-one 19-Nor-17alpha-ethynyl-17beta-hydroxy-4-androsten-3-one Norethisteron NORETHINDRONE norethisterone 17alpha-ethynyl-19-nor-4-androsten-17beta-ol-3-one 19-nor-17alpha-ethynyltestosterone 4-estren-17alpha-ethynyl-17beta-ol-3-one 17alpha-ethinyl-19-nortestosterone 17-ethynyl-17beta-hydroxyestr-4-en-3-one CHEMBL1162 17beta-hydroxy-19-norpregn-4-en-20-yn-3-one Micronor 11alpha-17beta-Dihydroxyandrost-1,4-dien-3-one, 11 BDBM91722
11alpha-17beta-Dihydroxyandrost-1,4-dien-3-one, 11 BDBM91722 CHEMBL229993 17beta-carbamoyloxy-3-sulfamoyloxyestra-1,3,5(10)-triene BDBM50219531
CHEMBL229993 17beta-carbamoyloxy-3-sulfamoyloxyestra-1,3,5(10)-triene BDBM50219531 17beta-Carbamoyloxy-2-methoxy-3-sulfamoyloxyestra-1,3,5(10)-triene BDBM50219532 CHEMBL389320
17beta-Carbamoyloxy-2-methoxy-3-sulfamoyloxyestra-1,3,5(10)-triene BDBM50219532 CHEMBL389320 CHEMBL385226 3-O-sulfamoylestradiol 17beta-O-(N,N-dipentyl)sulfamate BDBM50200942
CHEMBL385226 3-O-sulfamoylestradiol 17beta-O-(N,N-dipentyl)sulfamate BDBM50200942 2-methoxy-17beta-estradiol CHEMBL299613 BDBM50060957 Panzem 2-Hydroxyestradol 2-methyl ether
2-methoxy-17beta-estradiol CHEMBL299613 BDBM50060957 Panzem 2-Hydroxyestradol 2-methyl ether 2-OH-estradiol 2-hydroxy-17beta-estradiol cid_247304 (17beta)-estra-1,3,5(10)-triene-2,3,17-triol US10864248, Compound 2 2-OH-E2 BDBM50262140 CHEMBL467987 estra-1,3,5(10)-triene-2,3,17beta-triol
2-OH-estradiol 2-hydroxy-17beta-estradiol cid_247304 (17beta)-estra-1,3,5(10)-triene-2,3,17-triol US10864248, Compound 2 2-OH-E2 BDBM50262140 CHEMBL467987 estra-1,3,5(10)-triene-2,3,17beta-triol BDBM50292750 17beta-Hydroxy-11beta-[4-(methylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL1608 17beta-hydroxy-17alpha-propynyl-11beta-(4-N-methylaminophenyl)-estra-4,9-dien-3-one
BDBM50292750 17beta-Hydroxy-11beta-[4-(methylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL1608 17beta-hydroxy-17alpha-propynyl-11beta-(4-N-methylaminophenyl)-estra-4,9-dien-3-one 17beta-Estradiol-17-(beta-D-glucuronide) CHEMBL1697724 estradiol 17b glucuronide BDBM50344959 E17β-glucuronide
17beta-Estradiol-17-(beta-D-glucuronide) CHEMBL1697724 estradiol 17b glucuronide BDBM50344959 E17β-glucuronide BDBM50311133 17beta-[(N-Heptyl)methylamino]-4-methyl-4-aza-5alpha-androstan-3-one CHEMBL1080041
BDBM50311133 17beta-[(N-Heptyl)methylamino]-4-methyl-4-aza-5alpha-androstan-3-one CHEMBL1080041 CHEMBL1079512 17beta-[(N-Decyl)formamido]-4-methyl-4-aza-5alpha-androstan-3-one BDBM50311132
CHEMBL1079512 17beta-[(N-Decyl)formamido]-4-methyl-4-aza-5alpha-androstan-3-one BDBM50311132 CHEMBL490087 BDBM50292751 17beta-Hydroxy-11beta-[4-hydroxyphenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
CHEMBL490087 BDBM50292751 17beta-Hydroxy-11beta-[4-hydroxyphenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one 17-ethinyl-3,17-estradiol Ethinylestradiol CHEMBL691 17alpha-ethynylestra-1,3,5(10)-triene-3,17beta-diol ETHINYL ESTRADIOL Ethynyl estradiol 17alpha-Ethinyl estradiol 17alpha-ethynylestradiol 17-ethinyl-3,17-oestradiol ethinyloestradiol 17-ethinylestradiol BDBM50187243
17-ethinyl-3,17-estradiol Ethinylestradiol CHEMBL691 17alpha-ethynylestra-1,3,5(10)-triene-3,17beta-diol ETHINYL ESTRADIOL Ethynyl estradiol 17alpha-Ethinyl estradiol 17alpha-ethynylestradiol 17-ethinyl-3,17-oestradiol ethinyloestradiol 17-ethinylestradiol BDBM50187243 16-(Phenylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL560935 BDBM50296938
16-(Phenylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL560935 BDBM50296938 16alpha-(Methoxymethyl)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL538877 BDBM50296934
16alpha-(Methoxymethyl)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL538877 BDBM50296934 BDBM50296939 16-(Methylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL557116
BDBM50296939 16-(Methylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL557116 CHEMBL538137 16alpha-(Methyl)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296933
CHEMBL538137 16alpha-(Methyl)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296933 17beta-Hydroxy-11beta-[4-(5-carboxy-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292761 6-N-Methyl-N-[4'-[17beta-hydroxy-3-oxo-17alpha-(1-propynyl)-estra-4,9-dien-11beta-yl]-phenylaminohexanoicacid CHEMBL452860
17beta-Hydroxy-11beta-[4-(5-carboxy-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292761 6-N-Methyl-N-[4'-[17beta-hydroxy-3-oxo-17alpha-(1-propynyl)-estra-4,9-dien-11beta-yl]-phenylaminohexanoicacid CHEMBL452860 17beta-Hydroxy-11beta-[4-(9-bromnonoxy)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292752 CHEMBL454435
17beta-Hydroxy-11beta-[4-(9-bromnonoxy)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292752 CHEMBL454435 17beta-Hydroxy-11beta-[4-(N-methylhexylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292754 CHEMBL493845
17beta-Hydroxy-11beta-[4-(N-methylhexylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292754 CHEMBL493845 BDBM50292755 17beta-Hydroxy-11beta-[4-(N-methynonylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL459203
BDBM50292755 17beta-Hydroxy-11beta-[4-(N-methynonylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL459203 CHEMBL490088 BDBM50292753 17beta-Hydroxy-11beta-[4-(N-methylpropylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
CHEMBL490088 BDBM50292753 17beta-Hydroxy-11beta-[4-(N-methylpropylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL556229 BDBM50296935 16alpha-(3-Fluorobenzyl)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one
CHEMBL556229 BDBM50296935 16alpha-(3-Fluorobenzyl)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one 16-(Pyridin-3-ylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296942 CHEMBL562780
16-(Pyridin-3-ylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296942 CHEMBL562780 16-(Pyridin-4-ylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL556848 BDBM50296941
16-(Pyridin-4-ylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL556848 BDBM50296941 16-[(3,5-Difluorophenyl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL556431 BDBM50296937
16-[(3,5-Difluorophenyl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL556431 BDBM50296937 16-[(3-Fluorophenyl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296936 CHEMBL562471
16-[(3-Fluorophenyl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296936 CHEMBL562471 CHEMBL551269 16-(Pyrimidin-5-ylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296943
CHEMBL551269 16-(Pyrimidin-5-ylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296943 CHEMBL559485 BDBM50296940 16-(Pyridin-2-ylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one
CHEMBL559485 BDBM50296940 16-(Pyridin-2-ylmethylidene)-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL566118 5-[125I]Iodo-3'-O-(17beta-succinyl-5alpha-androstan-3-one)-2'-deoxyuridin-5'-yl Monophosphate BDBM50300786
CHEMBL566118 5-[125I]Iodo-3'-O-(17beta-succinyl-5alpha-androstan-3-one)-2'-deoxyuridin-5'-yl Monophosphate BDBM50300786 3-N-Methyl-N-[4-(11beta,17beta)-17-hydroxy-3-oxo-17alpha-(1-propynyl)estra-4,9-dien-11-yl]-phenylaminopropionic acid CHEMBL453382 BDBM50292760 17beta-Hydroxy-11beta-[4-(2-carboxy-N-methylethylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
3-N-Methyl-N-[4-(11beta,17beta)-17-hydroxy-3-oxo-17alpha-(1-propynyl)estra-4,9-dien-11-yl]-phenylaminopropionic acid CHEMBL453382 BDBM50292760 17beta-Hydroxy-11beta-[4-(2-carboxy-N-methylethylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one 17beta-Hydroxy-11beta-[4-(1-methyl-3-butylureido)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL453361 BDBM50292775
17beta-Hydroxy-11beta-[4-(1-methyl-3-butylureido)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL453361 BDBM50292775 17beta-Hydroxy-11beta-[4-(1-oxo-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292770 CHEMBL455176
17beta-Hydroxy-11beta-[4-(1-oxo-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292770 CHEMBL455176 17beta-Hydroxy-11beta-[4-(2-methoxycarbonyl-N-methylethylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292764 CHEMBL455175
17beta-Hydroxy-11beta-[4-(2-methoxycarbonyl-N-methylethylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292764 CHEMBL455175 17beta-Hydroxy-11beta-[4-(5-ethoxycarbonyl-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL504799 BDBM50292765
17beta-Hydroxy-11beta-[4-(5-ethoxycarbonyl-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL504799 BDBM50292765 17beta-Hydroxy-11beta-[4-(5-formyl-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL453381 BDBM50292759
17beta-Hydroxy-11beta-[4-(5-formyl-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL453381 BDBM50292759 17beta-Hydroxy-11beta-[4-(7-carboxy-N-methylheptylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292762 CHEMBL510869
17beta-Hydroxy-11beta-[4-(7-carboxy-N-methylheptylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292762 CHEMBL510869 17beta-Hydroxy-11beta-[4-(7-methoxycarbonyl-N-methylheptylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292766 CHEMBL499452
17beta-Hydroxy-11beta-[4-(7-methoxycarbonyl-N-methylheptylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292766 CHEMBL499452 BDBM50197371 17beta-Hydroxy-7a-{5-(4,4,5,5,5-pentafluoropentylsulfinyl)17b-Hydroxy-7a-{5-(4,4,5,5,5-pentafluoropentylsulfinyl)pentyl}-5a-androstan-3-one CHEMBL224736
BDBM50197371 17beta-Hydroxy-7a-{5-(4,4,5,5,5-pentafluoropentylsulfinyl)17b-Hydroxy-7a-{5-(4,4,5,5,5-pentafluoropentylsulfinyl)pentyl}-5a-androstan-3-one CHEMBL224736 BDBM50292757 17beta-Hydroxy-11beta-[4-(6-hydroxy-N-methylhexylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL455411
BDBM50292757 17beta-Hydroxy-11beta-[4-(6-hydroxy-N-methylhexylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL455411 BDBM50292767 CHEMBL450052 17beta-Hydroxy-11beta-[4-(10-methoxycarbonyl-N-methyldecylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
BDBM50292767 CHEMBL450052 17beta-Hydroxy-11beta-[4-(10-methoxycarbonyl-N-methyldecylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292768 CHEMBL445043 17beta-Hydroxy-11beta-[4-(5-carbamoyl-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
BDBM50292768 CHEMBL445043 17beta-Hydroxy-11beta-[4-(5-carbamoyl-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL444027 BDBM50292763 17beta-Hydroxy-11beta-[4-(10-carboxy-N-methyldecylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
CHEMBL444027 BDBM50292763 17beta-Hydroxy-11beta-[4-(10-carboxy-N-methyldecylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL454167 BDBM50292776 17beta-Hydroxy-11beta-[4-(1-methyl-3-butylthioureido)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
CHEMBL454167 BDBM50292776 17beta-Hydroxy-11beta-[4-(1-methyl-3-butylthioureido)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL489300 BDBM50292756 17beta-Hydroxy-11beta-[4-(3-hydroxy-N-methypropylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
CHEMBL489300 BDBM50292756 17beta-Hydroxy-11beta-[4-(3-hydroxy-N-methypropylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one 16-[(2-Methoxypyrimidin-5-yl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL559878 BDBM50296945
16-[(2-Methoxypyrimidin-5-yl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL559878 BDBM50296945 16-[(2-Methylpyrimidin-5-yl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296946 CHEMBL549375
16-[(2-Methylpyrimidin-5-yl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296946 CHEMBL549375 16-[(2-cyclopropylpyrimidin-5-yl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL551270 BDBM50296944
16-[(2-cyclopropylpyrimidin-5-yl)methylidene]-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL551270 BDBM50296944 17beta-Hydroxy-11beta-{4-[methyl-3-(4-chlorphenyl)-ureido]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292777 CHEMBL449900
17beta-Hydroxy-11beta-{4-[methyl-3-(4-chlorphenyl)-ureido]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292777 CHEMBL449900 BDBM50292778 17beta-Hydroxy-11beta-{4-[methyl-3-(4-chlorphenyl)-thioureido]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL507916
BDBM50292778 17beta-Hydroxy-11beta-{4-[methyl-3-(4-chlorphenyl)-thioureido]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL507916 17beta-Hydroxy-11beta-[4-(1-oxo-4-methoxycarbonyl-N-methylbutylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292773 CHEMBL449367
17beta-Hydroxy-11beta-[4-(1-oxo-4-methoxycarbonyl-N-methylbutylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292773 CHEMBL449367 17beta-Hydroxy-11beta-{4-[1-methyl-3-(5-methoxycarbonylpentyl)-ureido]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292780 CHEMBL451127
17beta-Hydroxy-11beta-{4-[1-methyl-3-(5-methoxycarbonylpentyl)-ureido]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292780 CHEMBL451127 BDBM50292774 17beta-Hydroxy-11beta-[4-(1-oxo-5-methoxycarbonyl-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL454224
BDBM50292774 17beta-Hydroxy-11beta-[4-(1-oxo-5-methoxycarbonyl-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL454224 BDBM50292779 17beta-Hydroxy-11beta-{4-[1-methyl-3-(5-carboxypentyl)-ureido]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL503056
BDBM50292779 17beta-Hydroxy-11beta-{4-[1-methyl-3-(5-carboxypentyl)-ureido]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL503056 CHEMBL500992 17beta-Hydroxy-11beta-[4-(1-oxo-4-carboxy-N-methylbutylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292771
CHEMBL500992 17beta-Hydroxy-11beta-[4-(1-oxo-4-carboxy-N-methylbutylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292771 CHEMBL505267 BDBM50292772 17beta-Hydroxy-11beta-[4-(1-oxo-5-carboxy-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
CHEMBL505267 BDBM50292772 17beta-Hydroxy-11beta-[4-(1-oxo-5-carboxy-N-methylpentylamino)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL563597 16-{[(2-Methylamino)pyrimidin-5-yl]methylidene}-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296947
CHEMBL563597 16-{[(2-Methylamino)pyrimidin-5-yl]methylidene}-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one BDBM50296947 16-{[(Pyridin-2-yl)pyrimidin-5-yl]methylidene}-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL562394 BDBM50296948
16-{[(Pyridin-2-yl)pyrimidin-5-yl]methylidene}-17beta-hydroxy-4-methyl-4-aza-5alpha-androst-1-en-3-one CHEMBL562394 BDBM50296948 BDBM50292758 CHEMBL507253 17beta-Hydroxy-11beta-{4-[5-(1,3-dioxol-2-yl)-N-methylpentylamino]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one
BDBM50292758 CHEMBL507253 17beta-Hydroxy-11beta-{4-[5-(1,3-dioxol-2-yl)-N-methylpentylamino]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292769 17beta-Hydroxy-11beta-[4-(1-methyl-7-oxo-1,8-diaza-dodecyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL505959
BDBM50292769 17beta-Hydroxy-11beta-[4-(1-methyl-7-oxo-1,8-diaza-dodecyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL505959 17beta-Hydroxy-11beta-[4-(1-methyl-6,9-dioxo-1-aza-5,10-dioxa-undecyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292787 CHEMBL506443
17beta-Hydroxy-11beta-[4-(1-methyl-6,9-dioxo-1-aza-5,10-dioxa-undecyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292787 CHEMBL506443 BDBM50292782 CHEMBL461357 17beta-Hydroxy-11beta-{4-[11-carboxy-1,13-dimethyl-2,9-dioxo-1,3,10-triaza-tetradecyl]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one
BDBM50292782 CHEMBL461357 17beta-Hydroxy-11beta-{4-[11-carboxy-1,13-dimethyl-2,9-dioxo-1,3,10-triaza-tetradecyl]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292788 17beta-Hydroxy-11beta-[4-(1-methyl-9,12-dioxo-1-aza-8,13-dioxa-tetradecyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL455530
BDBM50292788 17beta-Hydroxy-11beta-[4-(1-methyl-9,12-dioxo-1-aza-8,13-dioxa-tetradecyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL455530 17beta-estradiol (E2) 17α-ethinylestradiol Ovocyclin US9561238, E2 [2,4,6,7-3H]-17beta-estradiol CHEMBL135 (1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2,4,6-triene-5,14-diol US9034854, E2 CS336 [3H]]estradiol [2,4,6,7-3H]-E2 ESTRADIOL US9422324, E2 BDBM17292 [3H]-estradiol US9040509, E2 17 beta-Estradiol Estradiol-17 alpha
17beta-estradiol (E2) 17α-ethinylestradiol Ovocyclin US9561238, E2 [2,4,6,7-3H]-17beta-estradiol CHEMBL135 (1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2,4,6-triene-5,14-diol US9034854, E2 CS336 [3H]]estradiol [2,4,6,7-3H]-E2 ESTRADIOL US9422324, E2 BDBM17292 [3H]-estradiol US9040509, E2 17 beta-Estradiol Estradiol-17 alpha 17beta-Hydroxy-11beta-{4-[1-methyl-4-oxo-5-(4-carbamoyl-3-hydroxyphenyl)-1,5-diazapentyl]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292783 CHEMBL509832
17beta-Hydroxy-11beta-{4-[1-methyl-4-oxo-5-(4-carbamoyl-3-hydroxyphenyl)-1,5-diazapentyl]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292783 CHEMBL509832 CHEMBL446933 BDBM50292785 17beta-Hydroxy-11beta-[4-(9-hydroxy-1-methyl-6,9-dioxo-1-aza-5-oxa-nonyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
CHEMBL446933 BDBM50292785 17beta-Hydroxy-11beta-[4-(9-hydroxy-1-methyl-6,9-dioxo-1-aza-5-oxa-nonyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL451680 BDBM50292786 17beta-Hydroxy-11beta-[4-(12-hydroxy-1-methyl-9,12-dioxo-1-aza-8-oxa-dodecyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one
CHEMBL451680 BDBM50292786 17beta-Hydroxy-11beta-[4-(12-hydroxy-1-methyl-9,12-dioxo-1-aza-8-oxa-dodecyl)-phenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one BDBM50292784 CHEMBL504252 17beta-Hydroxy-11beta-{4-[1-methyl-6-(4-carbamoyl-3-hydroxyphenyl)-1-aza-5-oxa-hexyl]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one
BDBM50292784 CHEMBL504252 17beta-Hydroxy-11beta-{4-[1-methyl-6-(4-carbamoyl-3-hydroxyphenyl)-1-aza-5-oxa-hexyl]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one CHEMBL454121 BDBM50292781 17beta-Hydroxy-11beta-{4-[1,14,14-trimethyl-11-(2-methylpropyl)-2,9,12-trioxo-1,3,10-triaza-13-oxa-pentadecyl]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one
CHEMBL454121 BDBM50292781 17beta-Hydroxy-11beta-{4-[1,14,14-trimethyl-11-(2-methylpropyl)-2,9,12-trioxo-1,3,10-triaza-13-oxa-pentadecyl]-phenyl}-17alpha-(1-propinyl)-estra-4,9-dien-3-one (1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one Testosterone US9682960, Testosterone Testosterone, 1 BDBM8885 17beta-Hydroxyandrost-4-en-3-one
(1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one Testosterone US9682960, Testosterone Testosterone, 1 BDBM8885 17beta-Hydroxyandrost-4-en-3-one DHT Dihydrotestosterone [3H]DHT (5alpha,17beta)-17-hydroxyandrostan-3-one BDBM50366473 (1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-5-one BDBM18161 CHEMBL27769
DHT Dihydrotestosterone [3H]DHT (5alpha,17beta)-17-hydroxyandrostan-3-one BDBM50366473 (1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-5-one BDBM18161 CHEMBL27769 17beta-Hydroxy-11beta-[4-methoxyphenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one (8S,11R,13S,14S,17S)-17-hydroxy-11-(4-methoxy-phenyl)-13-methyl-17-prop-1-ynyl-1,2,6,7,8,11,12,13,14,15,16,17-dodecahydro-cyclopenta[a]phenanthren-3-one CHEMBL221686 BDBM50195153
17beta-Hydroxy-11beta-[4-methoxyphenyl]-17alpha-(1-propinyl)-estra-4,9-dien-3-one (8S,11R,13S,14S,17S)-17-hydroxy-11-(4-methoxy-phenyl)-13-methyl-17-prop-1-ynyl-1,2,6,7,8,11,12,13,14,15,16,17-dodecahydro-cyclopenta[a]phenanthren-3-one CHEMBL221686 BDBM50195153 BDBM50171448 CHEMBL190628 Sulfamic acid (11R,12S,15S,16S)-17-(S)-hydroxy-2-methoxy-13-methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-3-yl ester (9BETA,14BETA,17BETA)-17-HYDROXY-2-METHOXYESTRA-1,3,5(10)-TRIEN-3-YL SULFAMATE 2-methoxyestradiol-3-O-sulfamate
BDBM50171448 CHEMBL190628 Sulfamic acid (11R,12S,15S,16S)-17-(S)-hydroxy-2-methoxy-13-methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-3-yl ester (9BETA,14BETA,17BETA)-17-HYDROXY-2-METHOXYESTRA-1,3,5(10)-TRIEN-3-YL SULFAMATE 2-methoxyestradiol-3-O-sulfamate CHEMBL467212 (24R)-24-Adamantyl-1r,24-dihydroxy-2-methylene-22,23-didehydro-19,25,26,27-tetranorvitamin D3 BDBM50292706 (1R,3R,7E,17beta)-17-{(1R,2E,4R)-4-hydroxy-1-methyl-4-[(3S,5S,7S)-tricyclo[3.3.1.1~3,7~]dec-1-yl]but-2-en-1-yl}-2-methylidene-9,10-secoestra-5,7-diene-1,3-diol
CHEMBL467212 (24R)-24-Adamantyl-1r,24-dihydroxy-2-methylene-22,23-didehydro-19,25,26,27-tetranorvitamin D3 BDBM50292706 (1R,3R,7E,17beta)-17-{(1R,2E,4R)-4-hydroxy-1-methyl-4-[(3S,5S,7S)-tricyclo[3.3.1.1~3,7~]dec-1-yl]but-2-en-1-yl}-2-methylidene-9,10-secoestra-5,7-diene-1,3-diol (1R,3R,7E,17beta)-17-{(1S,2E,5R)-5-hydroxy-1-methyl-6-[(3S,5S,7S)-tricyclo[3.3.1.1~3,7~]dec-1-yl]hex-2-en-1-yl}-2-methylidene-9,10-secoestra-5,7-diene-1,3-diol CHEMBL452878 (25R)-26-adamantyl-1R,25-hydroxy-2-methylene-22,23-didehydro-19,27-dinor-20-epivitamin D3 BDBM50292704
(1R,3R,7E,17beta)-17-{(1S,2E,5R)-5-hydroxy-1-methyl-6-[(3S,5S,7S)-tricyclo[3.3.1.1~3,7~]dec-1-yl]hex-2-en-1-yl}-2-methylidene-9,10-secoestra-5,7-diene-1,3-diol CHEMBL452878 (25R)-26-adamantyl-1R,25-hydroxy-2-methylene-22,23-didehydro-19,27-dinor-20-epivitamin D3 BDBM50292704 [(8R,9S,13S,14S,17S)-3-hydroxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl] acetate acetic acid [(8R,9S,13S,14S,17S)-3-hydroxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl] ester [(8R,9S,13S,14S,17S)-13-methyl-3-oxidanyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl] ethanoate BDBM62878 cid_6852404 MLS000069774 SMR000058696 17BETA-ESTRADIOL 17-ACETATE
[(8R,9S,13S,14S,17S)-3-hydroxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl] acetate acetic acid [(8R,9S,13S,14S,17S)-3-hydroxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl] ester [(8R,9S,13S,14S,17S)-13-methyl-3-oxidanyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl] ethanoate BDBM62878 cid_6852404 MLS000069774 SMR000058696 17BETA-ESTRADIOL 17-ACETATE 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol(17beta-estradiol) (estradiol)13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol([6,7-3H]-estradiol) 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol(estradiol) CHEMBL135 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol(17alpha-estradiol) 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol 17beta-Estradiol 1,5-Dimethyl-2-phenyl-1,2-dihydro-pyrazol-3-one BDBM50005414 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol (estradiol) ESTRADIOL 15-methyltetracyclo[8.7.0.02,7.011,15]heptadeca-2,4,6-triene-5,14-diol Estradiol13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol( Estradiol)
13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol(17beta-estradiol) (estradiol)13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol([6,7-3H]-estradiol) 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol(estradiol) CHEMBL135 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol(17alpha-estradiol) 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol 17beta-Estradiol 1,5-Dimethyl-2-phenyl-1,2-dihydro-pyrazol-3-one BDBM50005414 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol (estradiol) ESTRADIOL 15-methyltetracyclo[8.7.0.02,7.011,15]heptadeca-2,4,6-triene-5,14-diol Estradiol13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol 13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol( Estradiol) Proscar BDBM50334788 (4aR,6aS,7S)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide CHEMBL710 FINASTERIDE 4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide(Finasteride) MK-906 (R)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (4aR,4bS,6aS,7S,9aS,9bS,11aR)-N-tert-butyl-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxamide Propecia (4aR,6aS,7S,11aR)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide US9061023, Finasteride (4aR,4bS,6aS,7S,9aS,9bS,11aR)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (4aR,4bS,6aS,9aS,9bS,11aR)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (4aR,6aS,11aR)-4a,6a-Dimethyl-2-oxo-hexadecahydro-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide 4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-androst-1-en-3-one 4a,6a,9a-Trimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid (Finasteride)
Proscar BDBM50334788 (4aR,6aS,7S)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide CHEMBL710 FINASTERIDE 4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide(Finasteride) MK-906 (R)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (4aR,4bS,6aS,7S,9aS,9bS,11aR)-N-tert-butyl-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxamide Propecia (4aR,6aS,7S,11aR)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide US9061023, Finasteride (4aR,4bS,6aS,7S,9aS,9bS,11aR)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (4aR,4bS,6aS,9aS,9bS,11aR)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (4aR,6aS,11aR)-4a,6a-Dimethyl-2-oxo-hexadecahydro-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide 4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid tert-butylamide (17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-androst-1-en-3-one 4a,6a,9a-Trimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid (Finasteride)